Watch a short video that provides an overview about the Global TB vaccine R&D roadmap
Global Roadmap for TB Vaccine R&D – Full version (PDF)
Global Roadmap for TB Vaccine R&D – Summary version (PDF)
Global Roadmap for TB Vaccine R&D – Background document (PDF)
Download the Global TB vaccine R&D roadmap



The Roadmap was launched at the Virtual Global Forum on TB Vaccines in April 2021, in partnership with AIGHD, during a special session attended by more than 250 participants, including researchers, developers, funders, regulators and other stakeholders.
In collaboration with the Tuberculosis Vaccine Initiative (TBVI), EDCTP has also been carrying out other activities to support projects planning TB vaccine trials and regulatory submissions. In 2021, a range of new tools were made available, including guidance on developing a plan for obtaining regulatory approval, guidance and a template to promote good data-management practices, and templates for developing clinical trial protocols related to the three priority needs identified by WHO (TB prevention in neonates/infants; prevention of active pulmonary disease in adults/adolescents; and use of a vaccine to treat TB in those already infected).
In addition, a registry has been developed providing information on clinical trial sites with the capacity to carry out TB vaccine trials in sub-Saharan Africa. The registry and guidance materials are all available on the EDCTP website.
scroll down
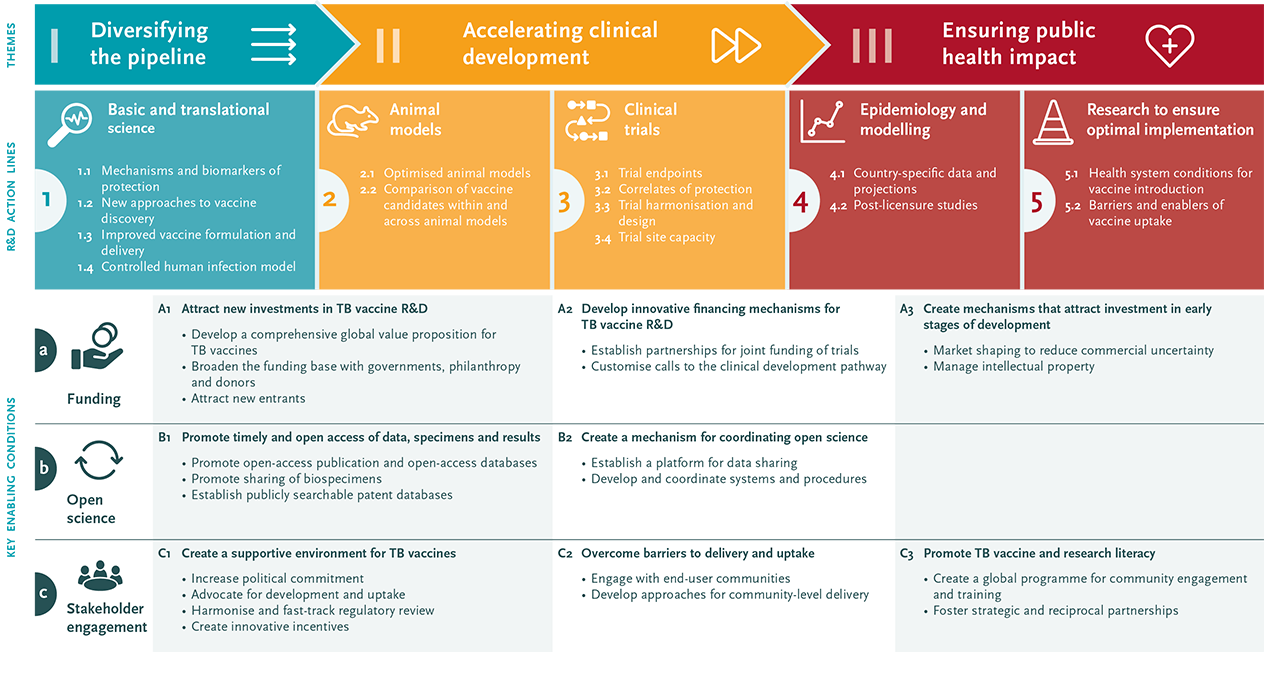
The consultation led to the identification of three priority areas: (1) diversifying the pipeline; (2) accelerating clinical development; and (3) ensuring public health impact. Three cross-cutting enabling factors were also identified: funding, open science and stakeholder engagement. The roadmap is available via the EDCTP website and a summary has been published in Lancet Infectious Diseases.
EDCTP is supporting multiple TB vaccine projects, including the POR TB consortium, priMe and MTBVAC. As candidate vaccines move towards large-scale trials, it is important that any potential obstacles to vaccine research and development (R&D), evaluation and implementation are identified and addressed.
In April 2021, EDCTP and the Amsterdam Institute for Global Health and Development (AIGHD) launched a Global Roadmap for research and development of TB vaccines. Developed through an iterative global consultative process and with close involvement of WHO, the Roadmap identifies the key barriers to TB vaccine R&D and implementation, and potential ways in which they might be overcome. It provides a shared set of priorities to guide the activities of all stakeholders with an interest in TB vaccine development and use.
EDCTP has worked closely with WHO on a new roadmap for TB vaccine development, and supported a range of activities to enhance coordination across TB vaccine studies.
Infographic: Tuberculosis vaccine R&D roadmap
Accelerating TB vaccine development

