

The use of rapid diagnostic tests could also lead to a significant change in case management in primary care, with treatment initiated without the need for parasitological analysis to confirm infection.
As well its contribution to Côte d’Ivoire’s success in 2021, the DiTECT-HAT project’s work will also be highly relevant to other countries targeting HAT elimination.
The project has also been assessing a potential approach for post-elimination monitoring, which will require testing on a population scale. Health workers have been carrying out house-to-house visits to collect blood on filter paper, which is then sent to a central laboratory for large-scale analysis. More than 13,000 people have been tested in Cote d’Ivoire, Burkina Faso, and in the Democratic Republic of the Congo, and analysis of samples is ongoing. The results will be used to identify the optimal approach to population-based post-elimination monitoring.
A further strand of the project is evaluating highly sensitive tests, including detection of parasite RNA, which could be used to track response to treatment in research studies and will be important in the development of new therapeutics.
Côte d’Ivoire’s submission to WHO included DiTECT-HAT’s passive case-detection activities. In addition, the country has expressed interest in the project’s post-elimination monitoring work, which could form part of its elimination validation/verification process up to 2030.
scroll down
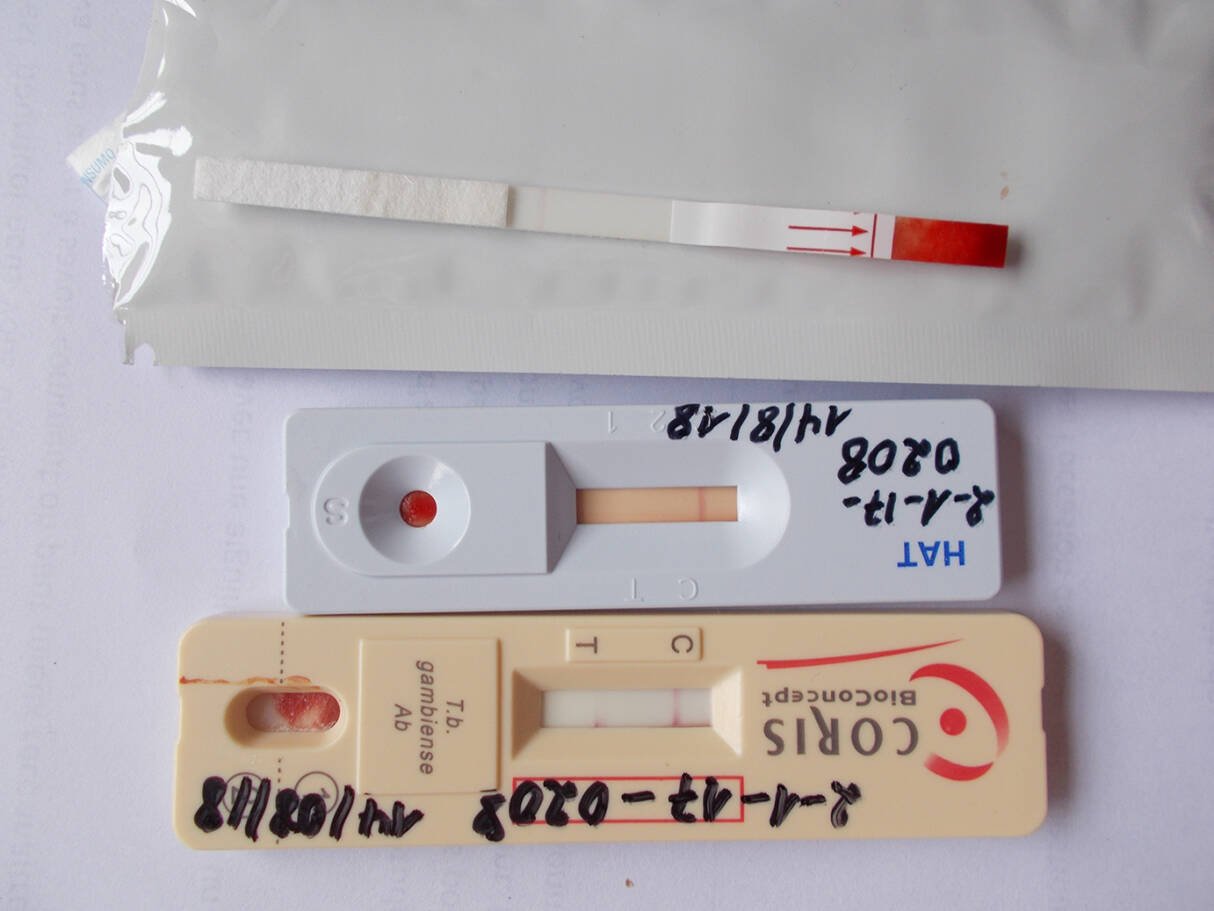
The DiTECT-HAT project has been evaluating three different testing strategies for HAT, each of which would have a distinct role to play in achieving and sustaining elimination. Achieving elimination will depend on the use of routine testing for HAT in healthcare facilities. The project has been evaluating three point-of-care rapid diagnostic tests and comparing results with laboratory-based methods, at sites in the Democratic Republic of the Congo, Côte d’Ivoire and Guinea. The Côte d’Ivoire studies identified tests suitable for use in the country, as well as symptoms associated with positive test results, which could act as a trigger for testing for HAT.
Enormous progress has been made in the control of human African trypanosomiasis (HAT), also known as sleeping sickness, with the disease earmarked for elimination as a public health threat. In 2021, WHO verified elimination in Côte d’Ivoire, an achievement that drew heavily on the work of the EDCTP-funded DiTECT-HAT project.
The DiTECT-HAT project has made key contributions to the WHO-certified elimination of human African trypanosomiasis (HAT) in Côte d’Ivoire.

Eliminating sleeping sickness in Côte d’Ivoire
Testing of dried blood spots in the regional reference lab.
Researchers working on the HAT rapid diagnostic test results.
Project: DiTECT-HAT study
Project lead: Dr Veerle Lejon, Institute of Research for Development, France
Countries involved: Belgium, Burkina Faso, the Democratic Republic of the Congo, Cote d’Ivoire, Guinea, United Kingdom
Year funded: 2016
EDCTP funding: €3 M
Grant agreement: DRIA2014-306
Project website: www.ditect-hat.eu
What comes next for the project?
Veerle Lejon: Analysis of the project results generated in Guinea and DRC will allow local test strategies to be refined. Furthermore, the inclusion of the cost of testing will provide information about the cost-efficiency of different potential test algorithms.
Through DiTECT-HAT, the project partners and personnel of health centres involved in the project have gained experience in conducting diagnostic trials. They are now already involved in diagnostic test evaluation of newly emerging rapid tests for HAT. The RNA specimens collected from treated HAT patients also allow evaluation of newly developed, potentially more sensitive RNA targets that would allow earlier detection of treatment failure.
Finally, diagnostic test performance determined in DiTECT-HAT can be used to check if tests comply with the use cases and the target product profiles for HAT defined by WHO.
Following project completion in early 2021, what project outputs have been further achieved (such as publications, progress towards PhDs and etc.)?
Veerle Lejon: The project has responded to questions that are relevant for control of HAT, such as what clinical signs and symptoms should trigger HAT testing, which rapid diagnostic tests are suitable for HAT screening, how can serological suspects in whom parasites cannot be detected microscopically be tested further, and what methods can be used to detect re-emergence of HAT. Furthermore, for the first time, a test may now be feasible to detect treatment failure, without having to perform a lumbar puncture, based on detection of trypanosomal RNA in the blood.
From a scientific point of view, so far six publications have been published on the project’s findings, while two additional manuscripts are close to finalisation or have been pre-submitted. Two PhD students from Burkina Faso and the Democratic Republic of the Congo (DRC) are finalising their PhD theses, and one student obtained his PhD in July at the University of Daloa in Côte d’Ivoire.
In 2021, the WHO validated elimination of human African trypanosomiasis (HAT) in Côte d’Ivoire, acknowledging the work of DiTECT-HAT in this achievement. How did the project contribute to HAT elimination in the country?
Veerle Lejon: The DiTECT-HAT project initiated the set-up of a passive surveillance network for HAT, integrated in the national health system of Côte d’Ivoire. Health personnel were trained to screen for HAT and rapid diagnostic tests were provided. The project identified five symptoms that should trigger HAT screening, and confirmed the appropriateness of the diagnostic test algorithm that is implemented in Côte d’Ivoire.
The health-seeking pathway of an Ivorian girl, with typical symptoms of HAT, who had consulted at least 20 health structures over 3 years before being diagnosed through DiTECT-HAT, illustrates the importance of such a passive surveillance system. In between, the network has been extended and includes also some reference hospitals outside the traditional HAT foci in Côte d’Ivoire.
Dr Michel Vaillant
(Luxembourg)

scroll down
Project Q&A


