
To inform the policy response to COVID-19, members of the PANDORA-ID-NET consortium have published more than 120 articles on COVID-19. These have examined multiple aspects of the detection, prevention, treatment and surveillance of COVID-19, as well as other key issues such as the impact of lockdown measures on women and children’s health and implications for other diseases of poverty, such as TB, HIV/AIDS and malaria.
PANDORA-ID-NET has also organised a range of online workshops focusing on laboratory quality control, as well as infection prevention and control and research ethics during epidemics. In partnership with The Global Health Network (TGHN), the consortium developed a PANDORA hub on the TGHN website, making available training materials and other resources.
In terms of practical activities, in partnership with two states in Nigeria, as well as the Nigeria Center for Disease Control (NCDC) and the Federal Ministry of Health of Germany, PANDORA-ID-NET deployed a mobile laboratory in Nigeria for identification of SARS-CoV-2 infections. In addition, PANDORA-ID-NET researchers received funding from the British Society for Microbial Chemistry to undertake a project on the impact of COVID-19 infection prevention and control measures on transmission of hospital-acquired infections and antimicrobial resistance in Africa.
At the same time, PANDORA-ID-NET continued its studies on other diseases, including a project in Uganda on surveillance of Crimean Congo haemorrhagic fever and studies on Lassa fever in Sierra Leone. The consortium also completed studies in Tanzania to determine the seroprevalence of chikungunya, dengue and Zika virus infections in diverse ecological zones.
Staying ALERRT
The next step will be a laboratory testing of samples collected across these countries. This will feed into a study, nested within FISSA, that is investigating risk factors and outcomes for COVID-19, with a particular focus on co-morbidities common in Africa, such as anaemia, helminth infections, malaria, HIV, TB and hepatitis.
ALERRT has also conducted a review of community engagement in sub-Saharan Africa during epidemics. Much of the evidence to date derives from the 2014–16 West African Ebola epidemic, and there is a need to extend the evidence base.
Part of ALERRT’s work has been carried out in collaboration with EDCTP Regional Networks of Excellence. ALERRT has a close relationship with the Eastern Africa Consortium for Clinical Research (EACCR). For example, the Uganda Virus Research Institute, part of EACCR, is participating in both the FISSA study and in the COVID-19 CCP. ALERRT has also collaborated with EACCR on several workshops and, with the Central African Network on Tuberculosis, HIV/AIDS and Malaria (CANTAM3) and PANDORA-ID-NET, jointly organised several workshops on laboratory technologies in 2021.
The ALERRT consortium has secured more than €10 million in additional funding, enabling more institutions across Africa to become involved in its activities. One particularly significant project, LASCOPE, funded by Wellcome and others, is systematically collecting data in Nigeria on key aspects of Lassa fever, such as clinical course, biological characteristics, case management and outcomes6. This will help to identify factors associated with severe disease and shed important light on disease mechanisms, to inform the development of much-needed new therapeutics.
The ALERRT consortium is carrying out a major study, FISSA (Febrile Illness in Sub-Saharan Africa), to better understand the causes, treatment and outcomes of fever in children and adults in widely spread sites across sub-Saharan Africa. In response to the pandemic, ALERRT revised the protocol of the FISSA study to include a research response component in the event of a declaration of a Public Health Emergency. In December 2021, ALERRT stopped recruitment into the FISSA study after enrolment of 8867 participants. The FISSA sample size is the largest of any fever study to date. Analysis is ongoing, and results are expected in the second quarter of 2022.
With funding from the Wellcome Trust and the UK Government, and working closely with the WHO Regional Office for Africa, Africa CDC and other networks across Africa, ALERRT set up the COVID-19 clinical characterisation protocol (CCP), which enables data and biological samples to be collected in a globally harmonised manner. The CCP study aims to understand the pathogen characteristics associated with virulence, the replication dynamics and in-host evolution of the pathogen, the dynamics of the host response, the pharmacology of antimicrobial or host-directed therapies, the transmission dynamics, and factors underlying individual susceptibility. As of December 2021, the consortium had retrospectively and prospectively recorded data from 10,225 patients in Cameroon, Democratic Republic of the Congo, Ghana, Guinea, Senegal and Uganda.
scroll down
The EDCTP-funded epidemic preparedness networks, ALERRT and PANDORA-ID-NET, were established to develop capacity for research studies during infectious disease outbreaks, and also to provide an infrastructure to support effective responses during outbreaks. As the COVID-19 pandemic struck, they rapidly pivoted to support regional COVID-19 response efforts, while also raising awareness of the impact of the pandemic on other infectious diseases.
EDCTP’s pandemic preparedness consortia, PANDORA-ID-NET and ALERRT, have been making important contributions to the fight against COVID-19 in Africa.

Networks unite against COVID-19

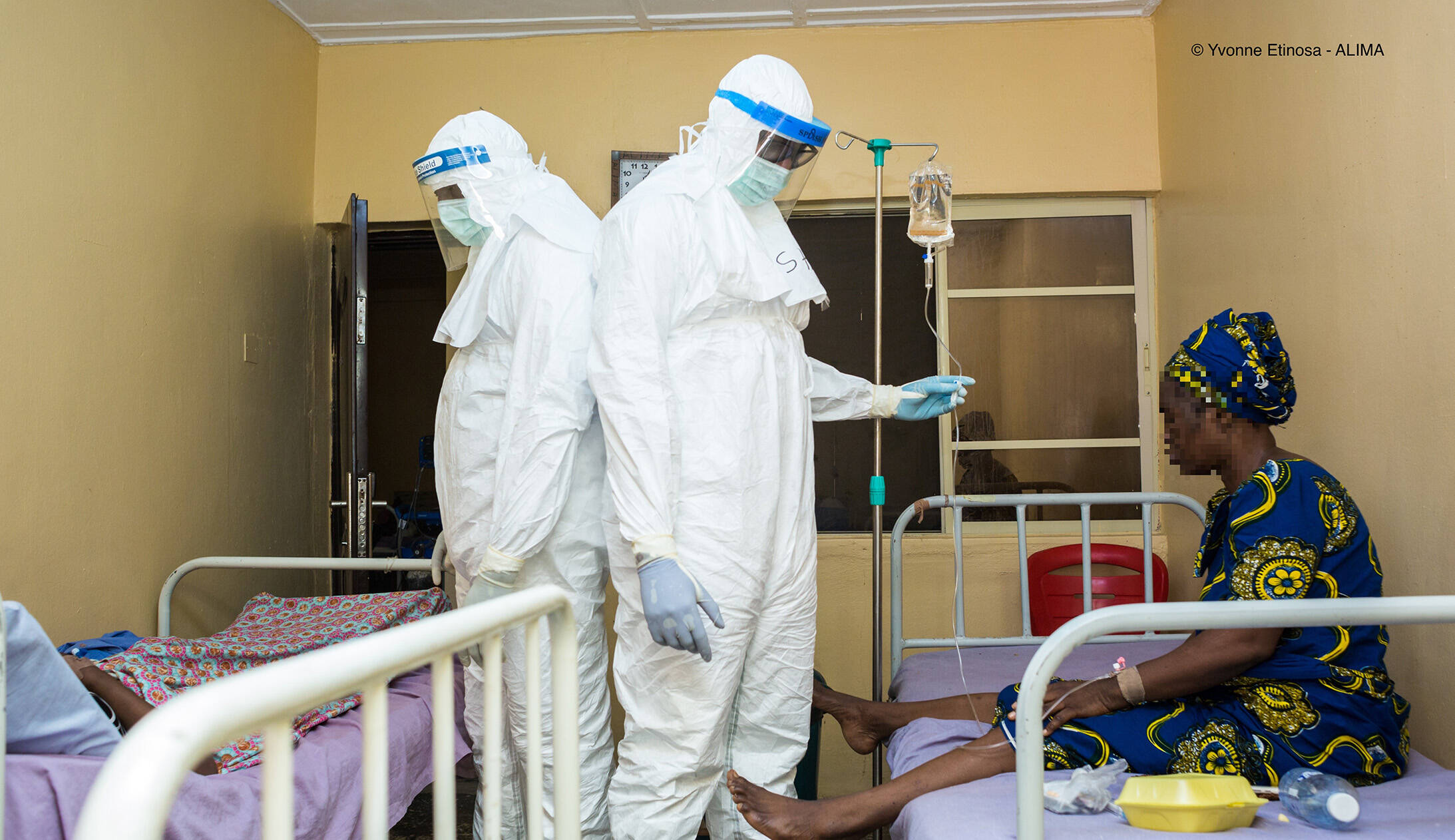
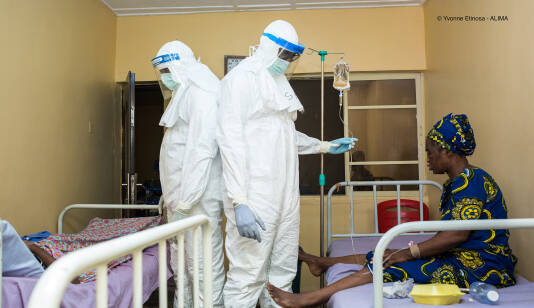
Researchers working on PANDORA-ID-NET activities.
UNZA and HerpeZ team collecting arenavirus samples in Zambia
Project: Pan-African Network for Rapid Research, Response, Relief and Preparedness for Infectious Disease Epidemics (PANDORA-ID-NET)
Project lead: Professor Francine Ntoumi, Fondation Congolaise pour la Recherche Médicale, Republic of Congo
Countries involved: Republic of Congo, France, Gabon, Germany, Ghana, Italy, Nigeria, Sierra Leone, Sudan, Tanzania, Uganda, United Kingdom, Zambia
Year funded: 2018
EDCTP funding: €10 M
Grant agreement: RIA2016E-1609
Project website: www.pandora-id.net
Professor Francine Ntoumi
(Republic of Congo)

What comes next for the Consortium?
Francine Ntoumi: The consortium is planning to conduct therapeutic and preventive clinical trials and to strengthen the data sharing of clinical data on local emerging and re-emerging infections.
With regard to the on-going monkeypox outbreak in Europe and Africa, PANDORA-ID-Net is actively engaged in preparedness and surveillance activities for health workers and researchers, including development of diagnostic tests.
Some of these activities were carried out in collaboration with other partners and networks. How did these collaborations impact the success of these activities? Please provide examples of partners and networks that have been key to PANDORA-ID-NET’s success.
Francine Ntoumi: During the pandemic, PANDORA-ID-Net partners shared protocols and reagents with regional and international organisations such as GISAID (the global influenza sequence database), Africa CDC, the Institute of Pathogen Genomics (IPG) and WHO AFRO.
Moreover, some specific and innovative studies were conducted. For example, the first African and European sentinel post-mortem whole-body autopsy studies of Italian and Zambian COVID-19 patients (hospital inpatients and community cases) were conducted. The Zambian team is training Congolese health workers to conduct such autopsies for infectious disease investigations.
In addition, with the support of Agence Universitaire Française (AUF), the Congolese team has worked on a 3D printed respirator. Other activities include development of event-based surveillance training modules for African Union Member States (collaboratively with Africa CDC) and development of a novel laboratory test kit for COVID-19 diagnosis that uses saliva samples - Irrua Specialist Teaching Hospital (ISTH), member institution of the PANDORA-ID-NET, in collaboration with Benson Idahosa and Ambrose Alli Universities in Nigeria.
Since the start of the COVID-19 pandemic, the Consortium has been very active and made important contributions to the fight against the disease in Africa. Could you please highlight some of the main achievements of the Consortium’s response to COVID-19?
Francine Ntoumi: All the PANDORA-ID-Net partners have been involved in the response to COVID-19 in their respective countries. Contributions were made to diagnostic, research, surveillance, treatment and prevention activities, as well as management of the national situation (PANDORA-ID-Net representatives being part of national response committees, the continental board of the Africa Centres for Disease Control and Prevention (Africa CDC) or WHO Regional Office for Africa (WHO AFRO)). All the partners who were trained and skilled in molecular biology, immunology and epidemiology were immediately in action in their respective countries in Europe and in Africa. Examples of activities included developing digital surveillance system for points of entry in Tanzania and investigating the social and economic impacts of COVID-19 lockdown in Sudan.
scroll down
Project Q&A
Prof. Ntoumi with members of her team.



ALERRT project staff members and volunteer (Lassa fever)
Member of the ALERRT research team.
Project: African Coalition for Epidemic Research, Response and Training (ALERRT)
Project lead: Professor Peter Horby, University of Oxford, United Kingdom
Countries involved: Belgium, Cameroon, Central African Republic, the Democratic Republic of the Congo, Cote d’Ivoire, France, Germany, Ghana, Madagascar, Senegal, Uganda, United Kingdom
Year funded: 2018
EDCTP funding: €10 M
Grant agreement: RIA2016E-1612
Project website: https://www.alerrt.global
Professor Peter Horby
(United Kingdom)

What comes next for the Consortium?
Peter Horby: In the heat of the COVID-19 pandemic, ALERRT demonstrated the value of a peer-to-peer African clinical research network that is collegial, agile, globally connected, and well prepared to respond to health emergencies. Beyond COVID-19, ALERRT partners are working together on other infectious disease health threats such as monkeypox and plague.
At our annual meeting in Entebbe in 2022, there was unanimous and enthusiastic support for continuing and developing the partnership, particularly to be able to quickly conduct drug, vaccine and diagnostics clinical trials when needed, and to strengthen the social science and implementation research components of our work to make it more impactful. We have committed to building on our strong foundations and securing ALERRT as a sustainable asset for Africa and the world.
Some of these activities were carried out in collaboration with other partners and networks. How did these collaborations impact the success of these activities? Please provide examples of partners and networks that have been key to ALERRT’s success.
Peter Horby: ALERRT is successful thanks to our 19 partner institutions, all of whom value the importance of policy engagement. The ALERRT Consortium has, therefore, worked closely with the WHO Regional Office for Africa (WHO AFRO) and the Africa Centres for Disease Control and Prevention (Africa CDC) to adapt the ISARIC Clinical Characterisation Protocol and to support the WHO in the roll-out of its clinical data platform in Africa and in the development of a protocol and case record form for studies on long COVID. ALERRT worked closely with TGHN in providing visibility for the tools and training materials developed. Collaboration with the African Academy of Sciences, WHO AFRO and the Africa CDC also helped develop documents and guidelines on research priorities during the pandemic.
Since the start of the COVID-19 pandemic, the Consortium has been very active and made important contributions to the fight against the disease in Africa. Could you please highlight some of the main achievements of the Consortium’s response to COVID-19?
Peter Horby: The ALERRT Consortium reacted quickly to the COVID-19 pandemic, adapting our Feasibility Assessment Form (or trigger form) to assess Africa’s COVID-19 research response needs and to determine how ALERRT could support those needs. An early action was to adapt the widely used ISARIC Clinical Characterisation Protocol (CCP) for COVID-19 in Africa. The contextualised CCP was used to rapidly collect standardised clinical information on almost 11,000 confirmed cases of COVID-19 in six ALERRT partner countries: Cameroon, the Democratic Republic of the Congo (DRC), Ghana, Guinea, Senegal and Uganda. The Africa-ready CCP tools developed by ALERRT, as well as training materials, were made freely available online via both the ALERRT and the Global Health Network (TGHN) websites. ALERRT also supported the WHO in collecting COVID-19 clinical characterisation data from some countries in Africa.
Four partner countries (Cameroon, Ghana, Senegal and Uganda) also surveyed the knowledge, practice and wellbeing of almost 9,000 healthcare workers during the COVID-19 pandemic, in what is thought to be the largest COVID-19 healthcare worker survey globally. ALERRT partners were also supported to join international drug clinical trials such as RECOVERY and ANTICOV. Some partners also used the capacity built to support vaccine clinical trials, such as the ongoing SARS-CoV-2 adjuvanted recombinant protein-based vaccine clinical trial led by Sanofi. In line with our training remit, 2,500 researchers and healthcare workers participated in COVID-19 workshops, covering more than 30 countries and 145 African institutions, with 1,500 certificates awarded.
scroll down
Project Q&A
Member of the ALERRT research team.