
More than 1000 babies and young children aged from 3 months to 6 years are being recruited. During 2021, the project achieved its 25% recruitment milestone. Among other activities, it also carried out a project examining the global scale of amoxicillin or co-amoxiclav use and associated costs, which revealed that co-amoxiclav accounts for a major proportion of total treatment costs in comparison to the scale of its use. This suggests that restricting co-amoxiclav use to severe cases could generate significant efficiency gains.
The project has also developed tools enabling the creation of more realistic ‘virtual populations’ of children for modelling studies of drug metabolism. Although WHO and others have generated standard growth charts for children, these may not be suitable for some populations, such as those who are malnourished, have HIV infection, or are affected by severe disease. Applied to WHO standards, the adjustments showed a better correlation with the data obtained from 1200 African children living with HIV. The methods will allow other groups to adjust existing standards to generate simulations that better represent actual populations being studied.
scroll down
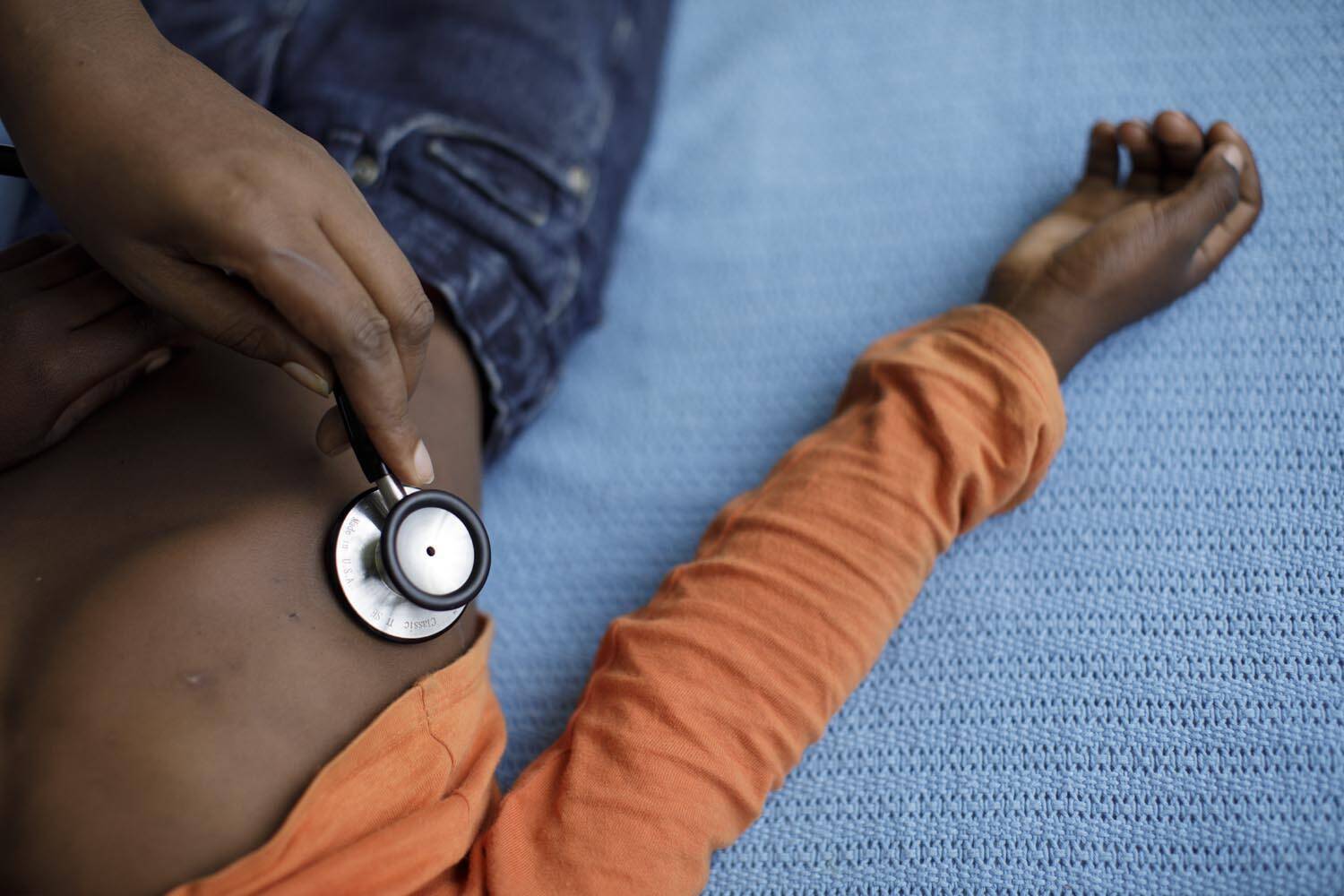
The PediCAP trial is assessing the potential of less intensive treatments, including the switch from injected to oral antibiotics, either amoxicillin or co-amoxiclav (a combination of amoxicillin with clavulanic acid).
The PediCAP team is using an innovative trial design to maximise the amount of useful evidence generated by the study. It is assessing different durations of treatment following the switch from injected antibiotics to amoxicillin or co-amoxiclav, and is monitoring side effects and the development of antibiotic resistance. It will also analyse whether the optimal type and duration of treatment depends on factors such as age or severity of pneumonia, which could enable treatment strategies to be further refined.
Pneumonia is the biggest global killer of children under five years of age, with at least half a million deaths estimated to occur in sub-Saharan Africa every year. For severe or very severe pneumonia, WHO recommends that children should be given injectable antibiotics for at least five days. However, this leads to long hospital stays, is costly, and increases the risk of hospital-acquired infections. Shorter and simpler treatments, particularly based on oral antibiotics, could therefore be beneficial to both patients and health systems.
The PediCAP trial, which hit its 25% recruitment milestone in 2021, is aiming to identify optimal treatments for young children hospitalised with pneumonia.

Optimising antibiotic treatments for young children
Project: PediCAP study
Project lead: Professor Carlo Giaquinto, Fondazione PENTA Onlus, Italy
Countries involved: Belgium, Italy, South Africa, Switzerland, Uganda, United Kingdom, Zambia, Zimbabwe
Year funded: 2018
EDCTP funding: €7 M
Grant agreement: RIA2017MC-2023
Project website: https://projectpedicap.org
Dr Julia Bielicki
(United Kingdom)

Prof. Carlo Giaquinto
(Italy)

What comes next for the project?
Kirsty Le Doare: We continue to focus on recruitment into the main trial and sub-studies, with strong contributions from all active sites and their satellites, aiming to complete recruitment in 2024. To promote even stronger interactions within the network, we are exploring the possibility of a face-to-face meeting in the near future hosted by a partner site – a welcome opportunity after several years without such a meeting due to the COVID-19 pandemic.
What progress was made in 2021 towards the project’s objectives?
Kirsty Le Doare: In 2021, PediCAP made progress in catching up on patient recruitment, which was substantially impacted by the COVID-19 pandemic in 2020. To maximise recruitment, several satellite sites to the partner sites were opened in 2021. This has also enabled us to progress or open the pharmacokinetic and microbiology sub-studies of PediCAP. Regular virtual interactions chaired by partner representatives in a rotating manner have consolidated the interactions within the network and have resulted in a collaboration to explore the impact of COVID-19 on the epidemiology of childhood severe acute respiratory tract infection in sub-Saharan Africa.
Antimicrobial resistance is a major global health threat. How is your project contributing or going to contribute to the control of the rising antibiotic resistance?
Kirsty Le Doare: Childhood pneumonia globally is still a major cause of mortality and morbidity, and is one of the most common infections for which children are admitted to hospital for treatment with antibiotics. While in hospital, children may become colonised with resistant bacteria that can cause difficult-to-treat infection in the future. Effective oral antibiotic treatment for severe paediatric pneumonia that can be given at home would reduce duration of inpatient stay and therefore the associated risks.
Furthermore, shorter treatment durations, also investigated in PediCAP, would reduce exposure to antibiotics themselves, further limiting selection of resistant bacteria in the children’s own microbiome.
The project has also developed tools enabling the creation of a more realistic ‘virtual population’ of children for modeling studies of drug metabolism. How do these tools work and why are they important?
Kirsty Le Doare: When investigating the relationship between drug levels (pharmacokinetics) and drug effects (pharmacodynamics), modelling and simulations are essential to overcome limitations of small datasets. In these models, key patient characteristics in the group of interest, such as weight, height, sex and age, have to be included to make sure that results are reliable. When simulations are done for children who are malnourished, children with HIV or acutely unwell children in resource-limited settings, typical ‘virtual’ patient populations are not representative, and the simulation results may therefore be unreliable.
As part of PediCAP, a reproducible workflow to construct a representative ‘virtual population’ of HIV-positive African children was constructed and tested. The method and virtual population have been shared as part of an open-access publication, and can therefore directly be used in modelling and simulation by other researchers interested in drug exposure and effects in similar groups of children.
The project is investigating less intensive treatment strategies based on a switch from injected to oral antibiotics in the management of childhood pneumonia. What are the advantages of this approach? Is the project likely to generate convincing evidence to support changes in prescribing guidelines?
Kirsty Le Doare: Current WHO recommendations for management of severe pneumonia are that children should be treated with a minimum of five days of injectable antibiotics. Since injectable antibiotics have to be given in hospital, children will have to be admitted for the entire treatment period, even when they do no longer require any other supportive hospital-based treatment. This has many downsides, including the risk of hospital-acquired infections and high costs. Oral antibiotics can be given at home when the child is otherwise stable. Studies done in high-income settings suggest that early oral antibiotic treatment is as good as injectable antibiotics, even in severe pneumonia. The innovative PediCAP trial is likely to generate robust evidence regarding the effectiveness of this approach in low- and middle-income countries.
scroll down
Project Q&A