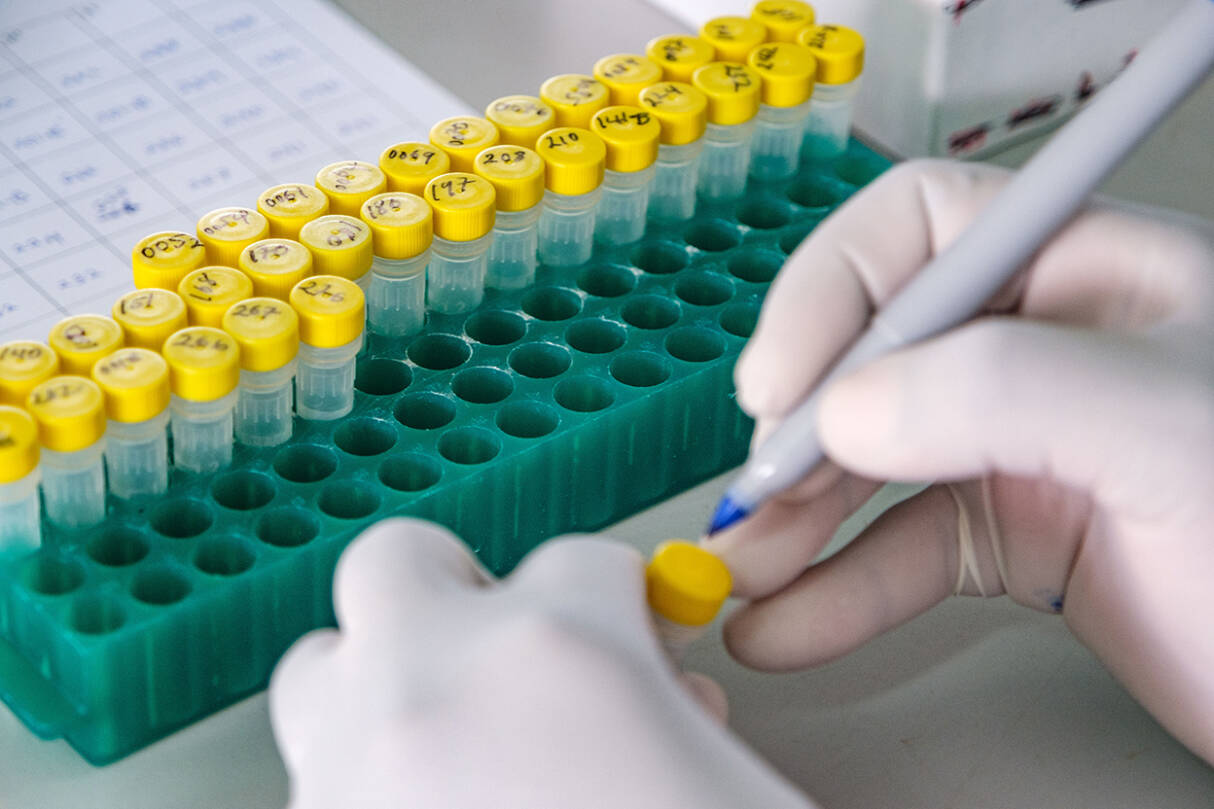
GBS disease is relatively rare, making clinical trials to assess efficacy difficult to carry out. The PREPARE consortium therefore aims to collect data on immune responses in vaccine recipients from sub-Saharan Africa and Europe, in order to identify ‘correlates of protection’ – elements of the immune response that are associated with protection against GBS infection. Such correlates of protection could be used to assess the likely efficacy of vaccines more easily, accelerating their clinical development.
The PREPARE team is also conducting studies to provide more information on the GBS disease burden, so the impact of vaccination can be better assessed. The team has also been carrying out research into perceptions of maternal vaccination against GBS among pregnant women, influential community representatives and healthcare workers in Uganda, to identify any potential barriers to the implementation of GBS vaccination in these key groups.
The PREPARE study also provided a platform for research into the impact of COVID-19 on pregnancy, immune responses in mothers and babies, and work with communities on infection control and prevention in pregnant women. The periCOVID Africa study, funded through EDCTP’s emergency COVID-19 call, is being carried out in The Gambia, Kenya, Malawi, Mozambique and Uganda and aims to collect data on 70,000 pregnancies.

scroll down
Control of GBS is likely to depend on safe and effective vaccines, administered to pregnant women. The PREPARE consortium is carrying out clinical trials of two promising candidate vaccines – GBS6, to be evaluated in Uganda, and GBS-NN/NN2, being tested in South Africa. The latter trial is recruiting pregnant women (with and without HIV infections) to assess safety and immunological responses in this key group. During 2021, recruitment into the GBS-NN/NN2 trial was completed.
Group B streptococci (GBS) are widely distributed bacteria that generally cause no ill-effects. However, during childbirth, there is a risk that they may be transmitted to newborn babies, potentially leading to severe pneumonia, sepsis or meningitis. Globally, more than 300,000 cases occur each year, leading to 57,000 stillbirths and 90,000 infant deaths.
The PREPARE project is collecting data on the prevalence of group B streptococcus, a leading neonatal infection in sub-Saharan Africa, and is evaluating vaccines to protect newborns against infection.

Vaccinating mothers to protect newborns
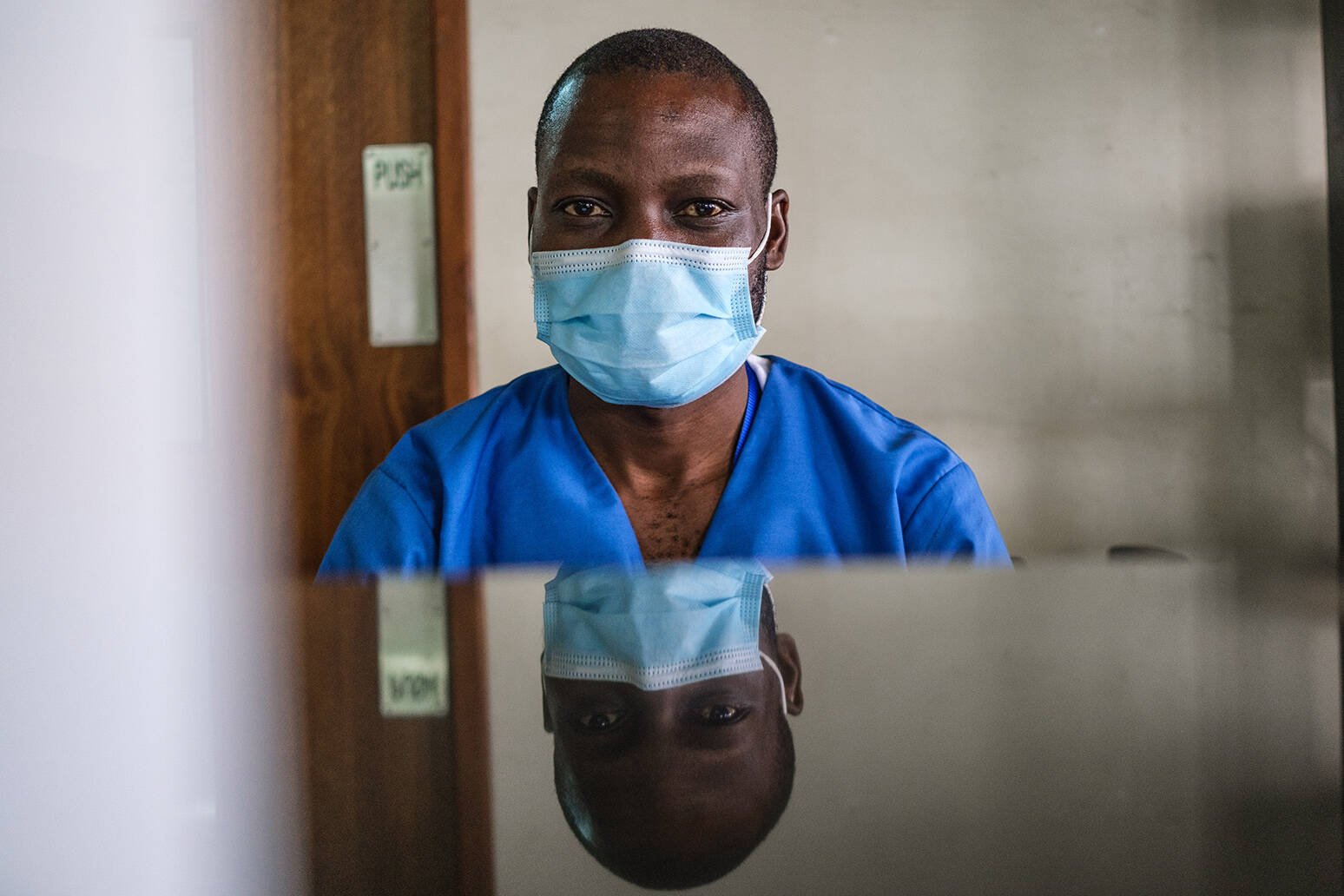
Dr Le Doare with her team.
Project: PREPARE study
Project lead: Dr Kirsty Le Doare, St George’s University of London, United Kingdom
Countries involved: Denmark, France, Italy, Malawi, the Netherlands, South Africa, Uganda, United Kingdom, United States
Year funded: 2019
EDCTP funding: €10 M
Grant agreement: RIA2018V-2304
Dr Kirsty Le Doare
(United Kingdom)

How does the PREPARE project connects to the periCOVID Africa project?
Kirsty Le Doare: The periCOVID Africa project combines the African sites from PREPARE (Malawi and Uganda) and the PRECISE consortium to provide information on protective antibodies against COVID-19 using the platform we developed through PREPARE. It is important to understand how much antibody protects women and infants from infection with COVID19 to allow us to measure vaccine responses as the pandemic progresses. A correlate of protection against COVID19 will help us to work out when and how often a pregnant woman needs to be vaccinated against COVID19 to keep their antibodies high enough to protect them and their babies.
What comes next for the project?
Kirsty Le Doare: We will launch the final GBS vaccine study in August 2022 and follow up women and their babies until they are 12 months old to look for any adverse events following vaccination. We will also complete our international study looking at the levels of antibody that protect against GBS infection. These studies will help us to understand whether we need different vaccine schedules for women living with HIV to protect mothers and babies from infection. The studies will also help to determine the amount of antibody that needs to cross the placenta to protect against infection (a correlate of protection) and this will be used to speed up licensure of the candidate GBS vaccines.
What progress was made in 2021 towards the project’s objectives?
Kirsty Le Doare: The Ugandan pregnancy registry, which details pregnancy outcomes, is now established so that we can track any adverse events following the introduction of a GBS vaccine. We continue to work with the communities in Uganda to increase vaccine confidence and the first two vaccine trials have completed recruitment.
The project is collecting data on the safety and efficacy of experimental vaccines against group B streptococci. Why is this important and how will you use the results?
Kirsty Le Doare: Group B streptococcus (GBS) is the leading cause of infection in the first months of life. We know that antibodies crossing the placenta provide protection, and over the past 30 years vaccines in pregnancy have been developed to boost this protection. Knowing how much antibody is needed to protect against infection, and if antibody behaves differently if a woman is living with HIV, will be vital to getting these vaccines licensed. If a vaccine is licensed, we could save nearly 400,000 infant lives each year.
scroll down
Project Q&A