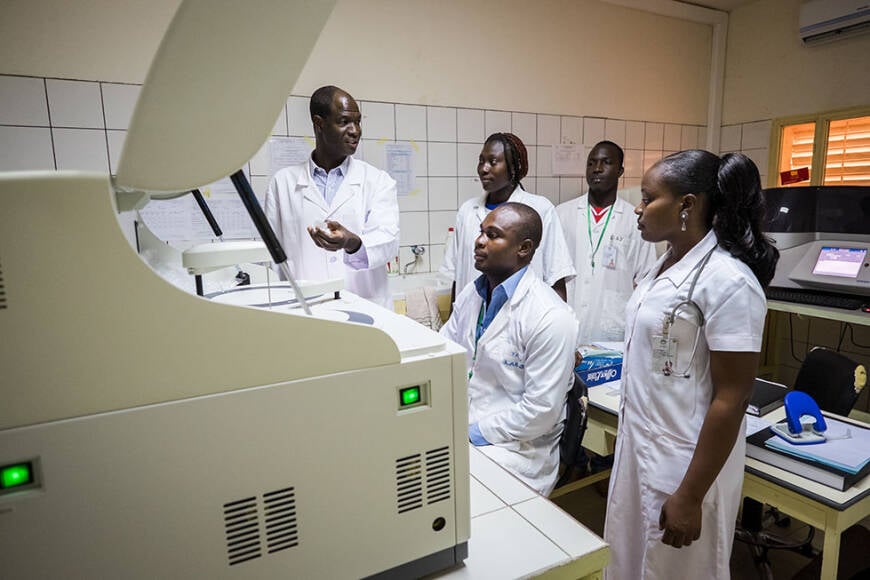
As well as supporting clinical research, EDCTP also funds projects that strengthen the capacity of countries, research institutions and individual researchers in sub-Saharan Africa to carry out high-quality clinical studies. The scope of this support is broad, including projects designed to strengthen ethics review mechanisms, regulatory systems, and drug safety monitoring, to protect research participants and maintain public confidence. Other important objectives are to build research capacity in countries that do not have a long tradition in clinical research, for example by linking of sites through Regional Networks of Excellence, and to lower the barriers to participation of female scientists in research.


Building capacity for drug safety monitoring


Spreading good practice


Sharing knowledge in global health research


Building ethics review and regulatory capacity