scroll down
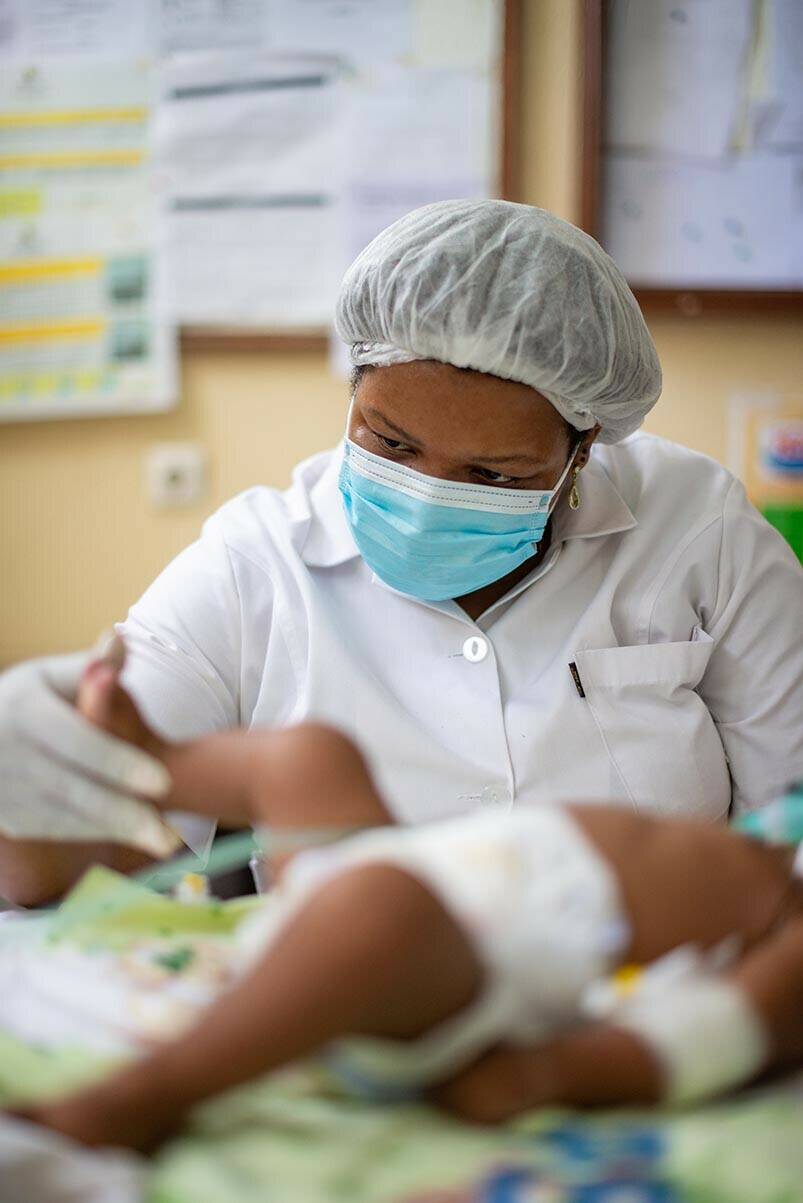
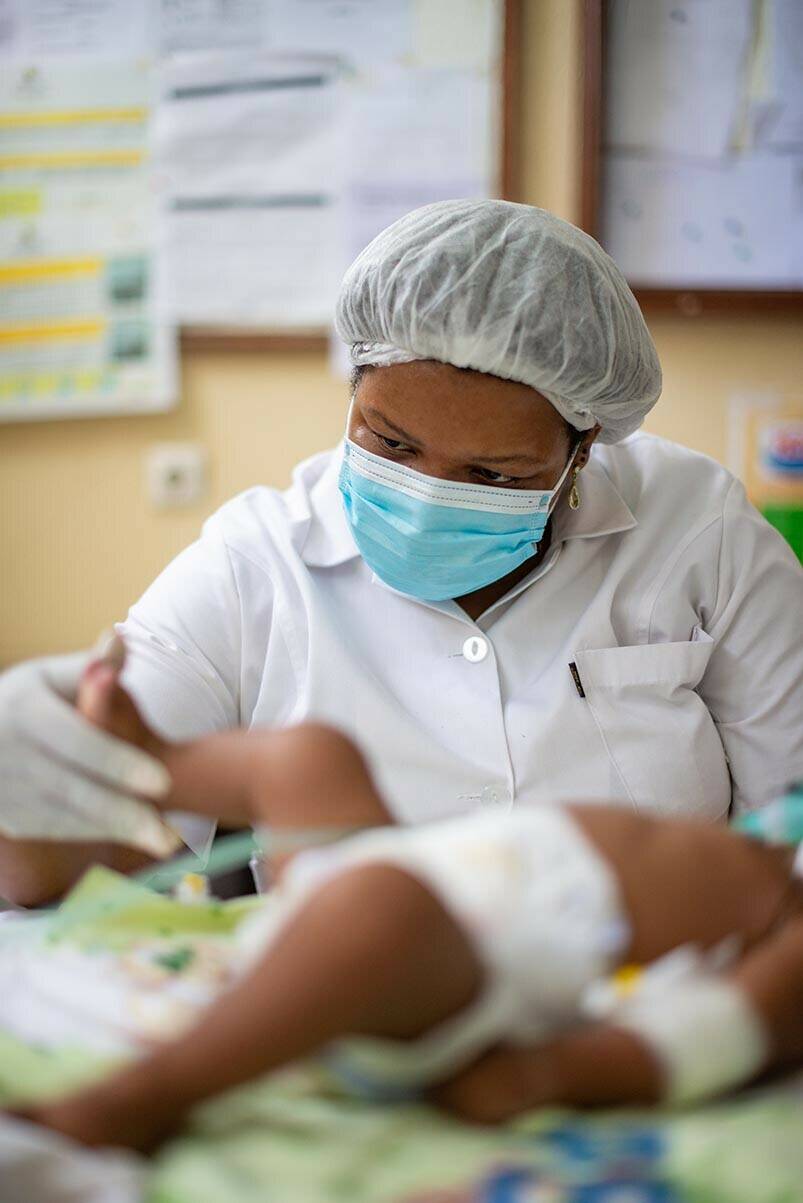
Enterotoxigenic E. coli (ETEC) is a common cause of diarrhoeal disease in many sub-Saharan African countries. The most advanced vaccine candidate against ETEC is a preparation of ETEC cells engineered to make large quantities of proteins likely to stimulate a strong immune response, alongside an adjuvant known as dmLT.
An EDCTP-funded phase IIb trial in The Gambia is evaluating the safety and efficacy of ETVAX in children. The project team is also developing a new formulation of ETVAX that will be better suited to young children, as well as a convenient device for administering the vaccine. In October 2022, the last participant in the ETEC ETVAX trial was vaccinated, with data analyses likely to be available in 2023.
If positive results are confirmed, the new all-in-one formulation will then be tested in an EDCTP-funded phase III trial in Zambia in infants 6–22 months in age.
The ETEC ETVAX phase IIb trial completed recruitment in 2022, with results due to feed into a pivotal phase III study of this much needed new vaccine.
Advancing ETVAX for ETEC
