
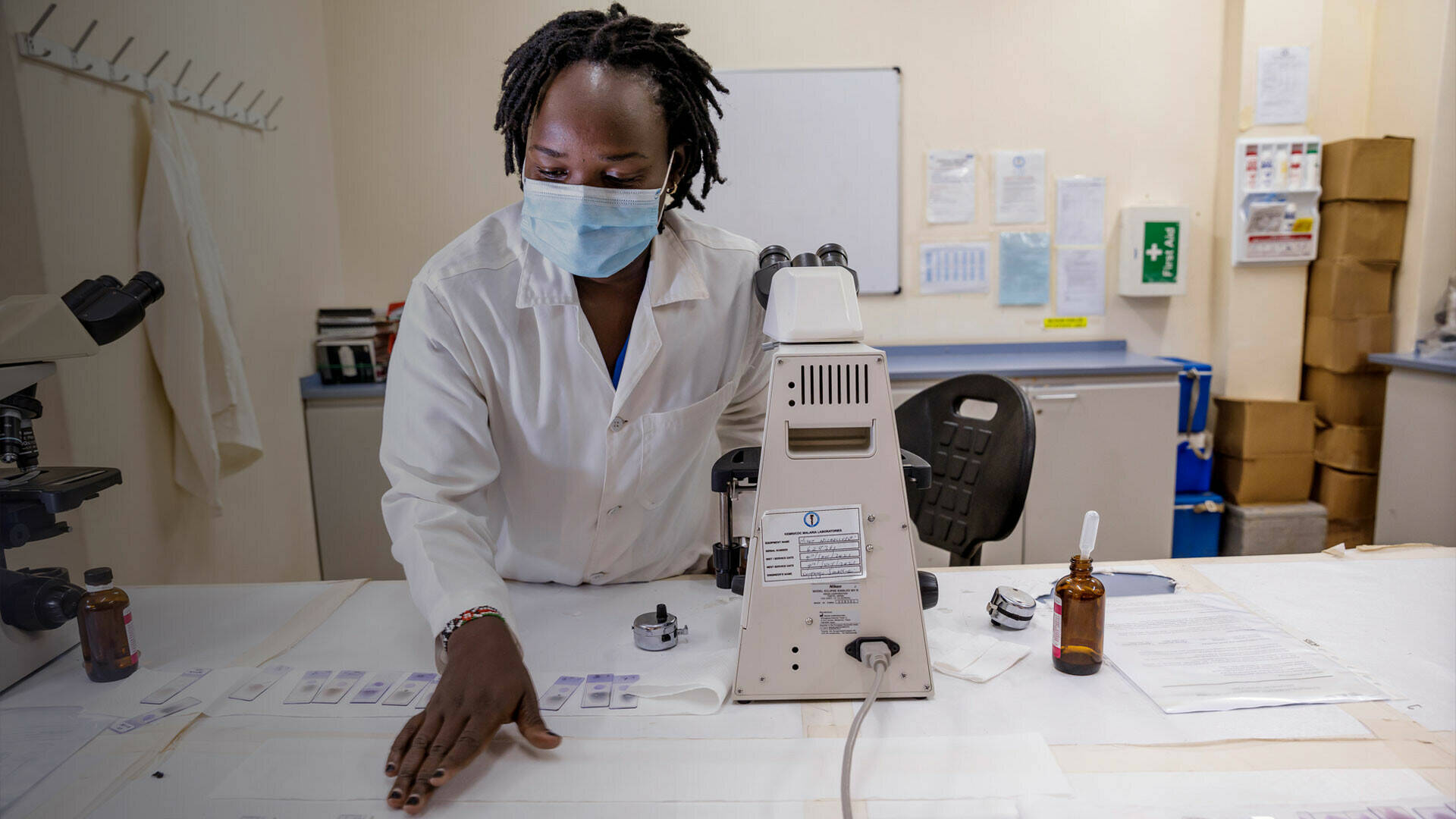
Strong ethics review and regulatory capacity is essential for ensuring that clinical research studies are ethically conceived and conducted, and monitored effectively. Regulatory capacity is also essential for monitoring and ensuring the safety of newly introduced medical interventions. EDCTP supports projects specifically designed to strengthen national ethics review and regulatory capacities, particularly through South–North and South–South collaborations.


Global ethical frameworks for research
Enhancing drug safety monitoring in Tanzania
Facilitating clinical research in Cameroon
Building ethics capacity in Portuguese-speaking countries
Building capacity in Ghana
Ethics capacity in West Africa
scroll down
Strong ethics review and regulatory capacity is essential for ensuring that clinical research studies are ethically conceived and conducted, and monitored effectively. Regulatory capacity is also essential for monitoring and ensuring the safety of newly introduced medical interventions. EDCTP supports projects specifically designed to strengthen national ethics review and regulatory capacities, particularly through South–North and South–South collaborations.
scroll down


Celebrating 20 years of impact through equitable partnerships
The European & Developing Countries Clinical Trials Partnership was established in 2003. This year, we will be celebrating 20 years of supporting equitable European-African research collaborations that have been, and still are, developing new and improved interventions against infectious diseases in sub-Saharan Africa. We invite you to join our Eleventh EDCTP Forum in Paris, France on 7-10 November 2023 and celebrate this momentous occasion with us.
Read the blog
EDCTP: A 20-year track record of supporting excellent, equitable research in Africa (23 June 2022, PLOS Global Public Health Global Health)
Case study 4
CHAPAS trials: Treatments for children with HIV
Case study 3
MVPE-CC: Ensuring malaria vaccine safety and effectiveness
Building capacity in Ghana
Ethics capacity in West Africa
As part of the 8th edition of the Science Summit around the 77th United Nations General Assembly (UNGA77), EDCTP organised the session ‘Funding research for impact: strengthening the role of science and evidence-based policymaking’. During this session, case studies were presented on advancing the production and usage of reliable research evidence in addressing unmet medical needs, especially amongst vulnerable populations in Africa.
EDCTP investments in R&D

In 435 projects awarded to date.
€814.45 M
Total funding
EDCTP is encouraging collaboration across TB vaccine projects in order to accelerate progress.
EDCTP has invested more than €50 million in four TB vaccine projects, focusing on prevention of infection in newborns (MTBVACN3, MTBVAC-Newborns, priMe) and prevention of recurrence in adults treated for TB disease (POR-TB). In April 2022, representatives of these projects met to exchange knowledge and experiences, at an event organised by the Tuberculosis Vaccine Initiative (TBVI), which has received funding from EDCTP to promote coordination across projects.
Although affected by the COVID-19 pandemic, the projects have continued to make progress. EDCTP, TBVI and the individual projects are exploring opportunities for collaboration, including on the development of new resources for the TB vaccine R&D community, building on those made available on the EDCTP website in 2021; these included various guidance documents and templates, as well as a directory of TB vaccine trial sites in Africa.
In May 2022, the MTBVACN3 project held its kick-off meeting in Spain. This phase III trial is evaluating a live attenuated Mycobacterium tuberculosis (Mtb) vaccine, MTBVAC, as a potential alternative or complement to the childhood vaccine BCG. It aims to recruit more than 7000 newborns at six sites in TB-endemic countries in sub-Saharan Africa, with participants being randomised to receive either MTBVAC or BCG.
The MTBVACN3 project is drawing on the related MTBVAC-Newborns trial, a phase IIa dose-escalation study which completed recruitment in 2021. This work identified the dose being evaluated in the MTBVACN3 project.
Independently, in 2022 the priMe project completed recruitment into its phase III trial of VPM1002. Like BCG, VPM1002 is based on a relative of Mtb, M. bovis, which causes bovine TB, but has been precisely engineered so that it retains some of the immune-stimulating components that have been lost in BCG. The priMe trial is being carried out in Gabon, Kenya, South Africa, Tanzania and Uganda, with VPM1002 also being compared with BCG.



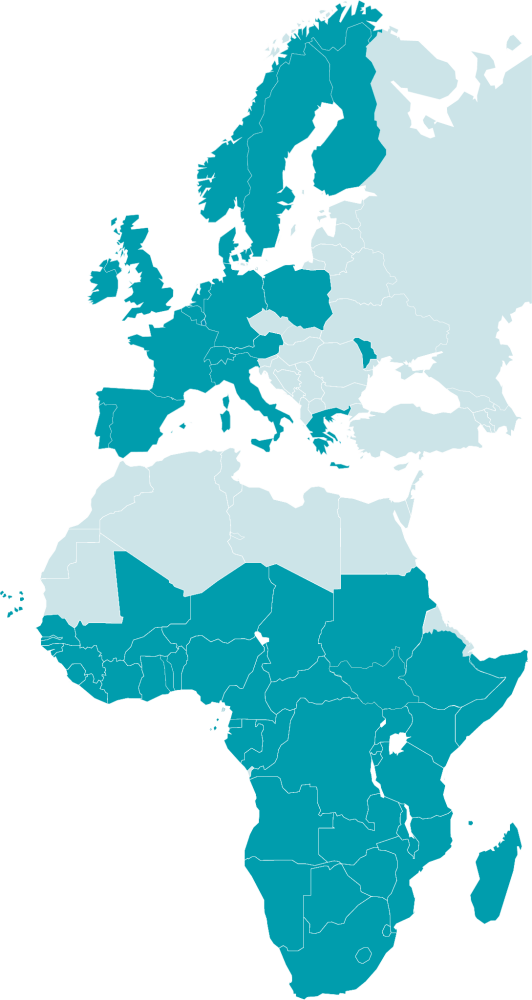
19 European countries
and 44 African countries
63 countries participate in EDCTP-funded activities:
Participation in EDCTP activities
(indicative amounts based on signed grant agreements and proposals approved for funding)
Cumulative investment to call for proposals by year
Investment to call for proposals by year
Investments in calls for proposals
€155.63 M
€16.85 M
€83.42 M
€112.14 M
€814.45 M
€100.27 M
€255.90 M
€444.04 M
€608.47 M
€720.37 M
€94.08 M
2014
2015
2016
2017
2018
2019
2020
€164.19 M
€188.14 M

Facts & figures
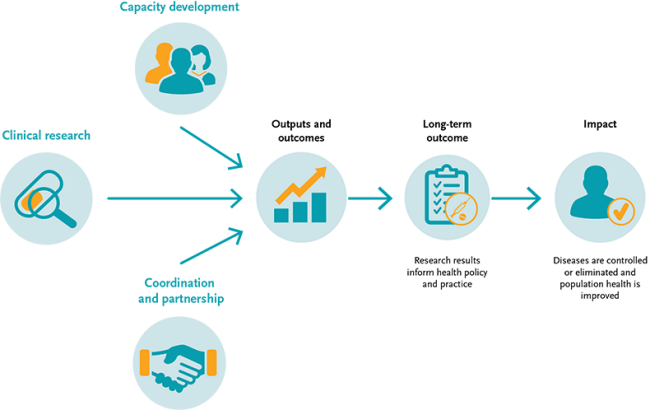
By May 2022, the second EDCTP programme (EDCTP2; 2014-2024) portfolio comprised 435 grants awarded through 60 calls for proposals, representing a total investment of EUR 814 million. Clinical trials supported by EDCTP2 involve international collaborations spanning >60 countries and 350 institutions in Europe and sub-Saharan Africa, with broader global collaboration. Results from these clinical trials have generated pivotal evidence which has informed national and international policy and practice.
Despite substantial investment in the development of new diagnostics, vaccines and drugs for poverty-related diseases, these interventions may not reach their target populations or be used to their full potential. This is particularly the case in sub-Saharan Africa, where health systems are generally not optimal nor adequately prepared for the delivery and uptake of new or improved products and interventions. Therefore, EDCTP is very intentional in promoting translation of policy-relevant research results into policy and practice.