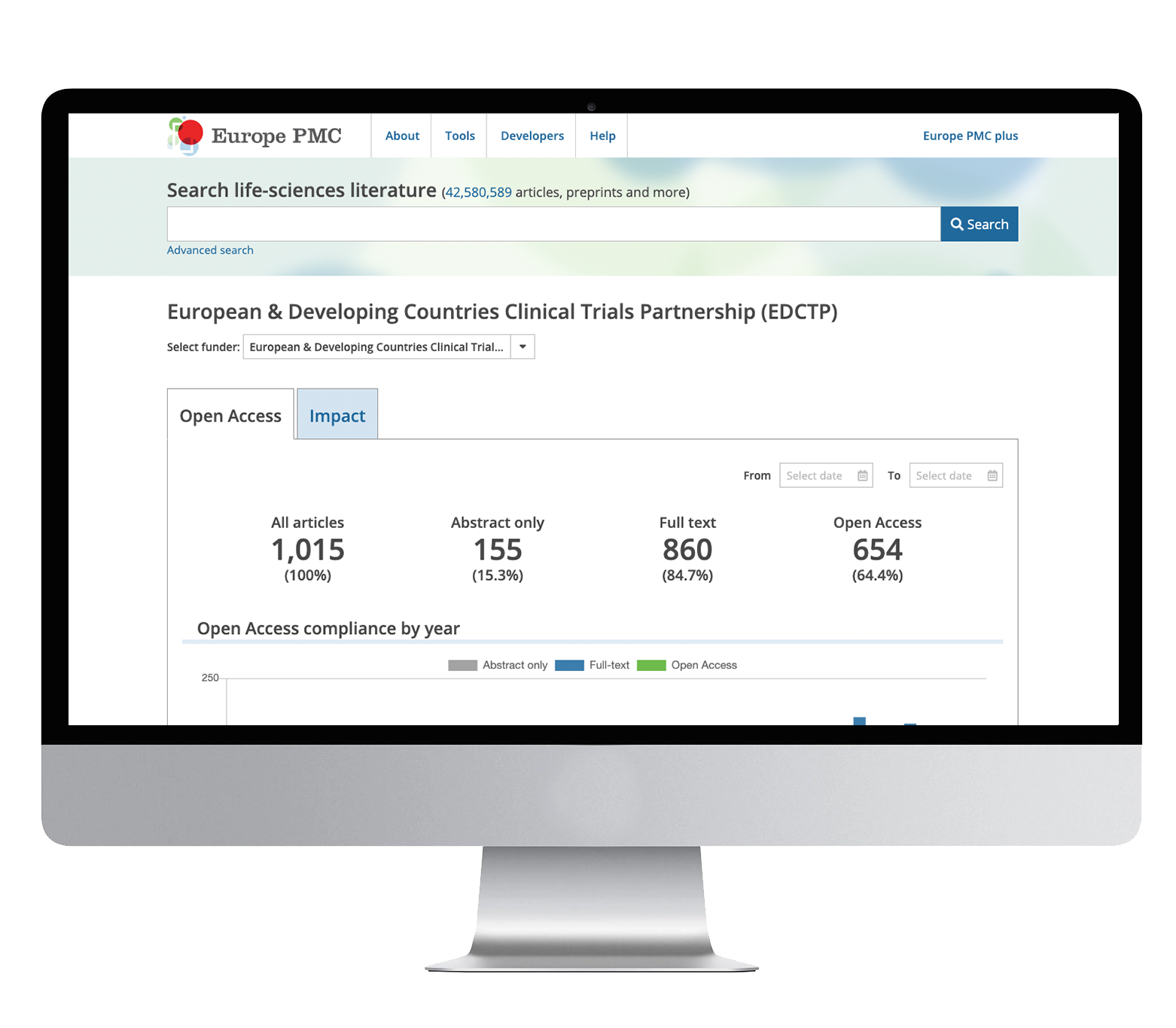
In 2022, EDCTP became a member of Europe PMC (PubMed Central), an open science platform that maintains a worldwide collection of scientific articles and other research outputs. Joining Europe PMC enables EDCTP-funded researchers to share their publications via one central location and ensures that they also satisfy the EDCTP requirement for publications to be openly accessible as soon as possible.
In March 2022, EDCTP arranged a training webinar for grantholders to introduce the EDCTP open-access policy and to provide step-by-step guidance on how to comply with the policy and deposit research manuscripts in Europe PMC.
The EDCTP Knowledge Hub was featured in new WHO guidance on sharing of research data, published in April 2022. The guidance outlines WHO’s position that sharing research data is a global public good. The policy requires WHO-supported researchers to share data in ways that are equitable, ethical and efficient, and follow the ‘FAIR’ principles – that data are findable, accessible, interoperable and reusable. This position is in keeping with EDCTP’s policy on clinical trials registration, publication and data sharing.
WHO’s guide also directs researchers to comprehensive resources, including the EDCTP Knowledge Hub. According to the guidance document, the Knowledge Hub “provides a wealth of good research practice resources, reading material and explanatory videos as well as a search function to identify the best online repository to share your research data”.

scroll down
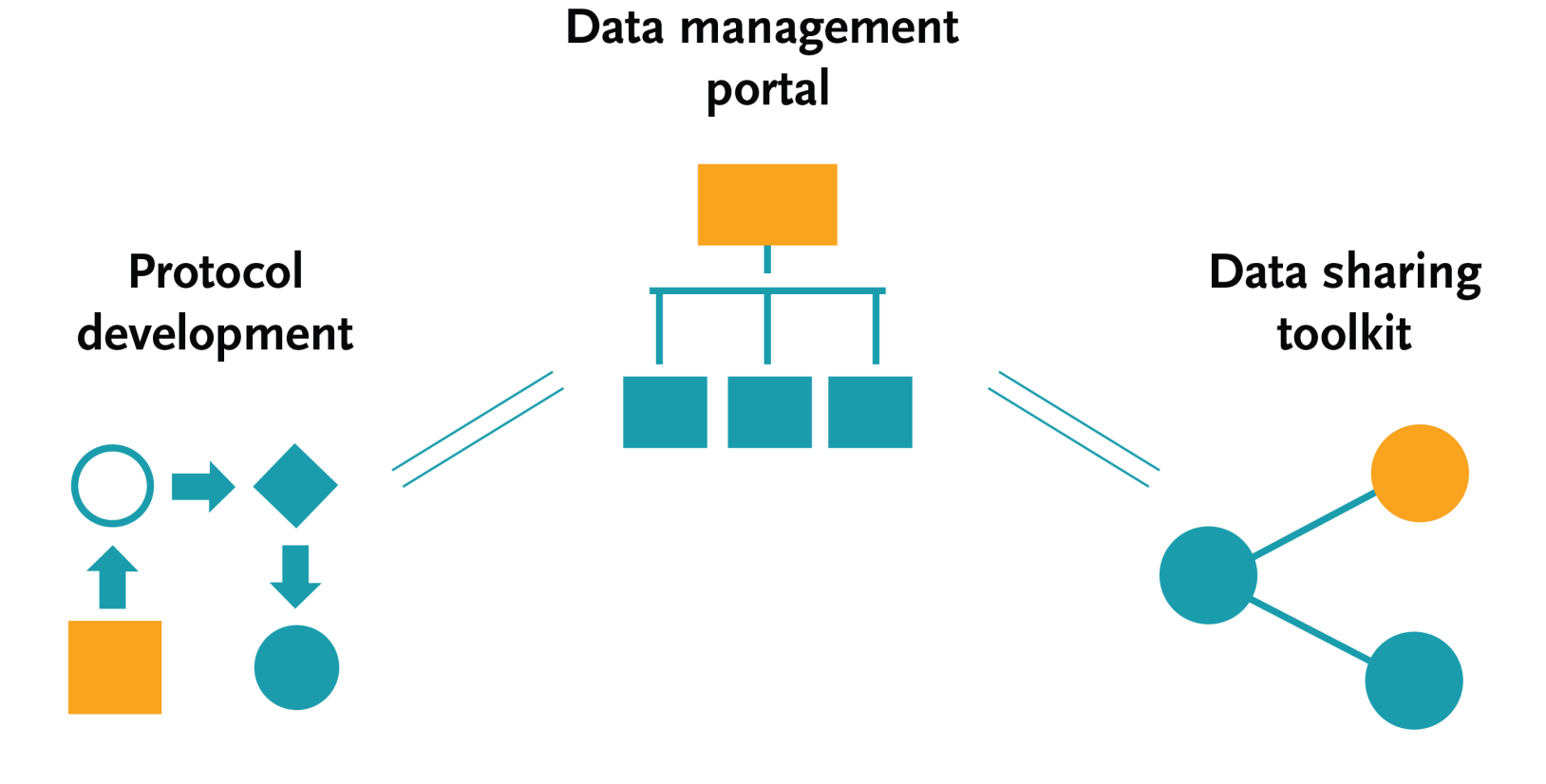
An article published in the journal Trials in 2022 summarised development of the EDCTP Knowledge Hub, an online platform that provides access to a range of essential tools to guide the planning and implementation of high-quality clinical studies. Developed in collaboration with the Global Health Network (TGHN), the Knowledge Hub is designed to address some of the key barriers to clinical protocol development in low- and middle-income countries.
The Knowledge Hub includes a range of resources to help researchers develop research questions into a protocol and to adopt gold-standard clinical data-management practices. It includes interactive and comprehensive toolkits covering the essential steps of a clinical health research study. It also raises awareness of the importance of data sharing, and provides practical advice and guidance on how to share data.
The EDCTP Knowledge Hub, an online resource for those planning clinical studies, has been praised in new WHO guidance, while EDCTP has also joined Europe PMC (PubMed Central) and encourages its researchers to submit articles to this open-access repository.

Sharing knowledge
