Case study 2
Project: PREPARE study
Project lead: Dr Kirsty Le Doare, St George's University of London, United Kingdom
Countries involved: Denmark, France, Italy, Malawi, the Netherlands, South Africa, Uganda, United Kingdom, United States
Year funded: 2019
EDCTP funding: €10 M
Grant agreement: RIA2018V-2304
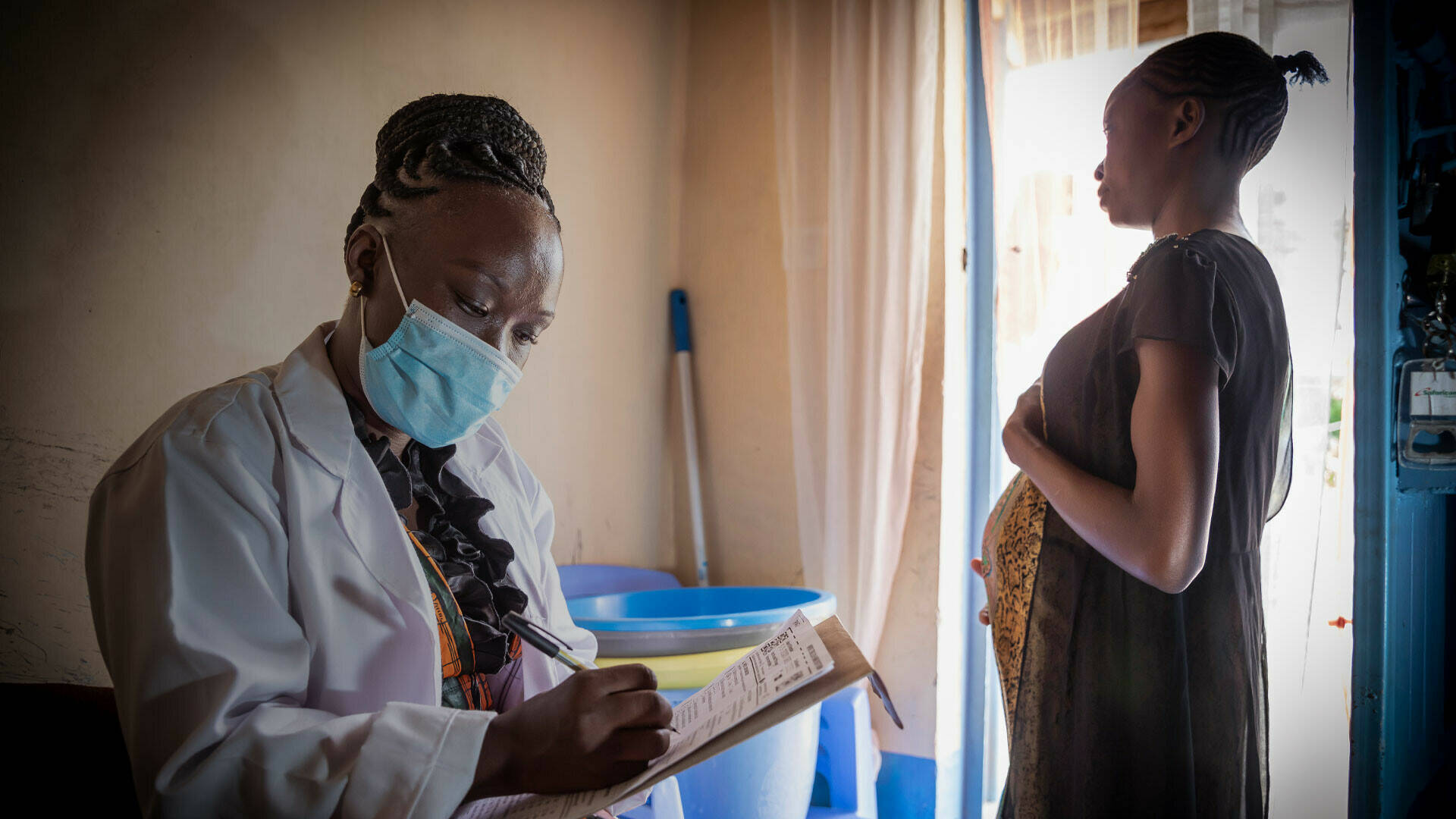
Group B Streptococcus (GBS) is a leading cause of neonatal infection (including pneumonia, sepsis and meningitis) in Europe, and increasingly recognised as a significant cause of neonatal infection in sub-Saharan Africa.
Control of GBS is likely to depend on safe and effective vaccines, administered to pregnant women. The PREPARE consortium is carrying out clinical trials of two promising candidate vaccines: the GBS6, to be evaluated in Uganda), and the GBS-NN/NN2, being tested in in Uganda and South Africa. The trial that uses the MinervaX GBS-NN/NN2 vaccine candidate aims to evaluate the safety and reactogenicity of the vaccine candidate in pregnant women. Both trial sites in South Africa and Uganda have recently completed recruitment.
The PREPARE study also provided a platform for research into the impact of COVID-19 on pregnancy, immune responses in mothers and babies, and work with communities on infection control and prevention in pregnant women. The periCOVID Africa study, funded through EDCTP’s emergency COVID-19 call, is being carried out in The Gambia, Kenya, Malawi, Mozambique and Uganda and aims to collect data on 70,000 pregnancies.
scroll down

scroll down
Case study 2

Group B Streptococcus (GBS) is a leading cause of neonatal infection (including pneumonia, sepsis and meningitis) in Europe, and increasingly recognised as a significant cause of neonatal infection in sub-Saharan Africa.
Control of GBS is likely to depend on safe and effective vaccines, administered to pregnant women. The PREPARE consortium is carrying out clinical trials of two promising candidate vaccines: the GBS6, to be evaluated in Uganda), and the GBS-NN/NN2, being tested in in Uganda and South Africa. The trial that uses the MinervaX GBS-NN/NN2 vaccine candidate aims to evaluate the safety and reactogenicity of the vaccine candidate in pregnant women. Both trial sites in South Africa and Uganda have recently completed recruitment.
The PREPARE study also provided a platform for research into the impact of COVID-19 on pregnancy, immune responses in mothers and babies, and work with communities on infection control and prevention in pregnant women. The periCOVID Africa study, funded through EDCTP’s emergency COVID-19 call, is being carried out in The Gambia, Kenya, Malawi, Mozambique and Uganda and aims to collect data on 70,000 pregnancies.
Project: PREPARE study
Project lead: Dr Kirsty Le Doare, St George's University of London, United Kingdom
Countries involved: Denmark, France, Italy, Malawi, the Netherlands, South Africa, Uganda, United Kingdom, United States
Year funded: 2019
EDCTP funding: €10 M
Grant agreement: RIA2018V-2304