Impact of EDCTP-supported activities on policy and practice
Unnecessary delay or failure to translate research findings into policy and practice prevents research from achieving maximum public health benefit. Therefore, EDCTP is very intentional in promoting translation of policy-relevant research results into policy and practice.

Celebrating 20 years of impact through equitable partnerships
The European & Developing Countries Clinical Trials Partnership was established in 2003. This year, we will be celebrating 20 years of supporting equitable European-African research collaborations that have been, and still are, developing new and improved interventions against infectious diseases in sub-Saharan Africa. We invite you to join our Eleventh EDCTP Forum in Paris, France on 7-10 November 2023 and celebrate this momentous occasion with us.
Read the blog
EDCTP: A 20-year track record of supporting excellent, equitable research in Africa (23 June 2022, PLOS Global Public Health Global Health)
Case study 4
CHAPAS trials: Treatments for children with HIV
Case study 3
MVPE-CC: Ensuring malaria vaccine safety and effectiveness
Case study 2
PREPARE and periCOVID Africa: Vaccinating mothers to protect newborns and generating data on COVID-19 infections in pregnant women and their offspring
Case study 1
AMBITION-cm: Simpler and safer treatment of cryptococcal meningitis
Case studies
As part of the 8th edition of the Science Summit around the 77th United Nations General Assembly (UNGA77), EDCTP organised the session ‘Funding research for impact: strengthening the role of science and evidence-based
policymaking’. During this session, case studies were presented on advancing the production and usage of reliable research evidence in addressing unmet medical needs, especially amongst vulnerable populations in Africa.

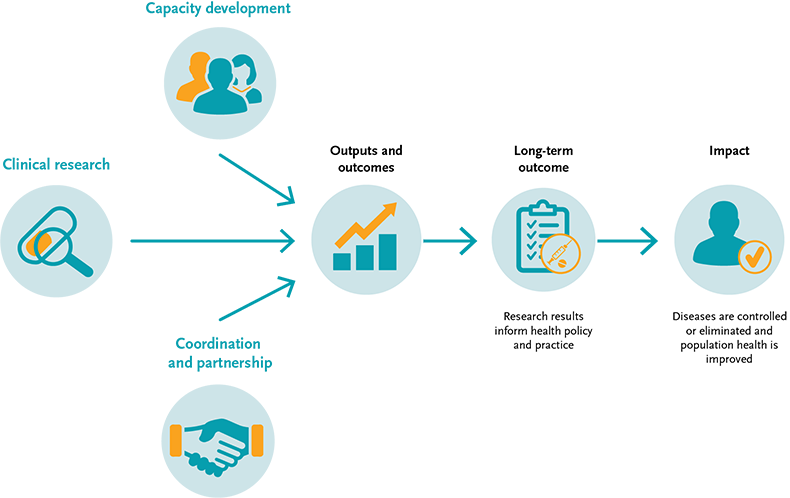
Monitoring & evaluation
In line with internationally recognised principles of results-based management, we monitor and evaluate the EDCTP2 programme to report on its operational performance and results. Our monitoring and evaluation approach draws on a ‘theory of change’ model (see figure below) that maps out the route through which our funding generates immediate outputs (such as the published results from clinical trials), outcomes (such as changes in health policy and practice) and impact (improvements in health and wellbeing and economic gain). This approach helps collect and analyse information from our grants and other activities that are of strategic importance, and more importantly to justify good value-for-money investments.
EDCTP investments in R&D

In 435 projects awarded to date.
€814.45 M
Total funding



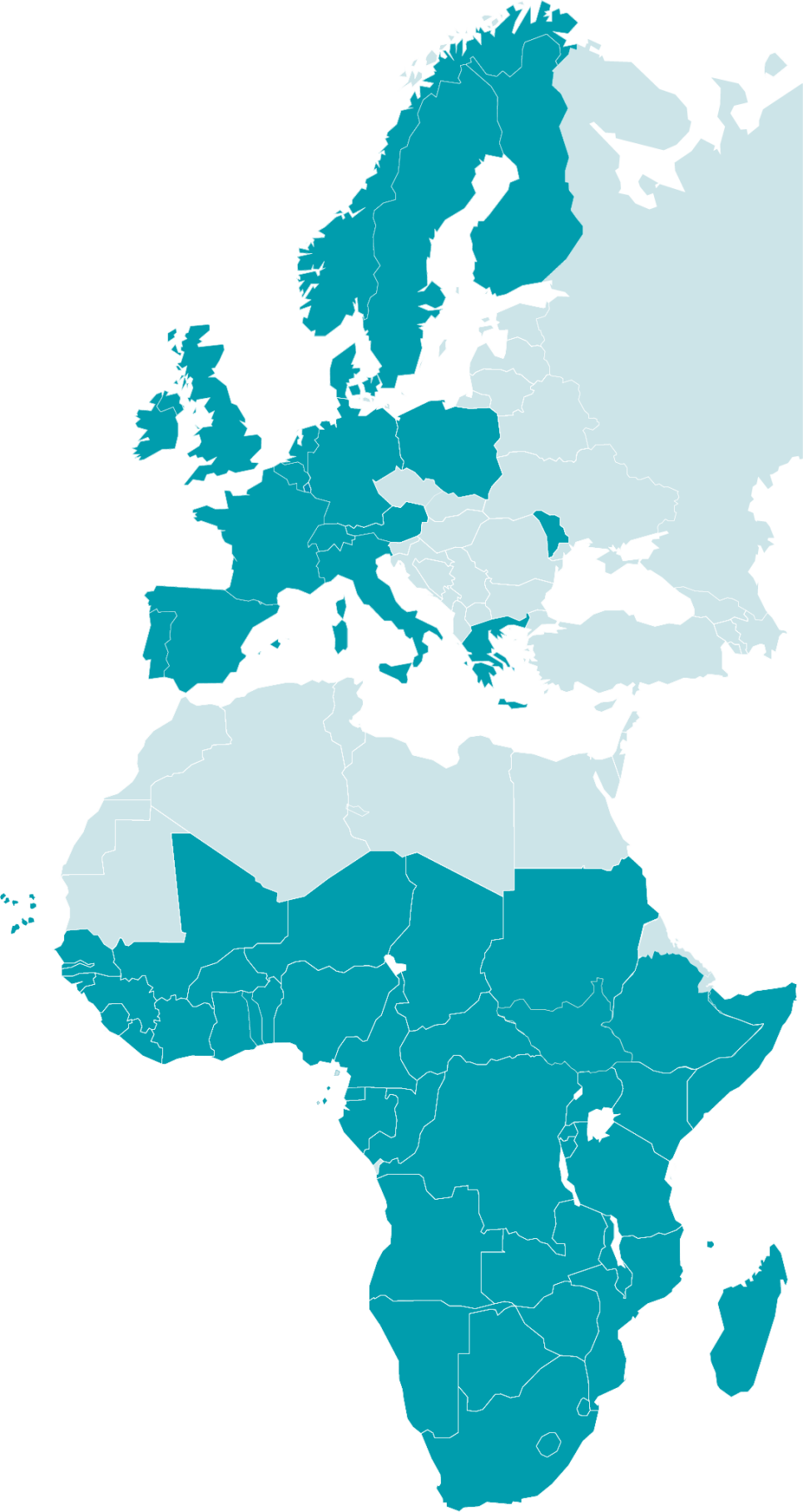
19 European countries
and 44 African countries
63 countries participate in EDCTP-funded activities:
Participation in EDCTP activities
(indicative amounts based on signed grant agreements and proposals approved for funding)
Cumulative investment to call for proposals by year
Investment to call for proposals by year
Investments in calls for proposals
€155.63 M
€16.85 M
€83.42 M
€112.14 M
€814.45 M
€100.27 M
€255.90 M
€444.04 M
€608.47 M
€720.37 M
€94.08 M
2014
2015
2016
2017
2018
2019
2020
€164.19 M
€188.14 M
Facts & figures
By May 2022, the second EDCTP programme (EDCTP2; 2014-2024) portfolio comprised 435 grants awarded through 60 calls for proposals, representing a total investment of EUR 814 million. Clinical trials supported by EDCTP2 involve international collaborations spanning >60 countries and 350 institutions in Europe and sub-Saharan Africa, with broader global collaboration. Results from these clinical trials have generated pivotal evidence which has informed national and international policy and practice.
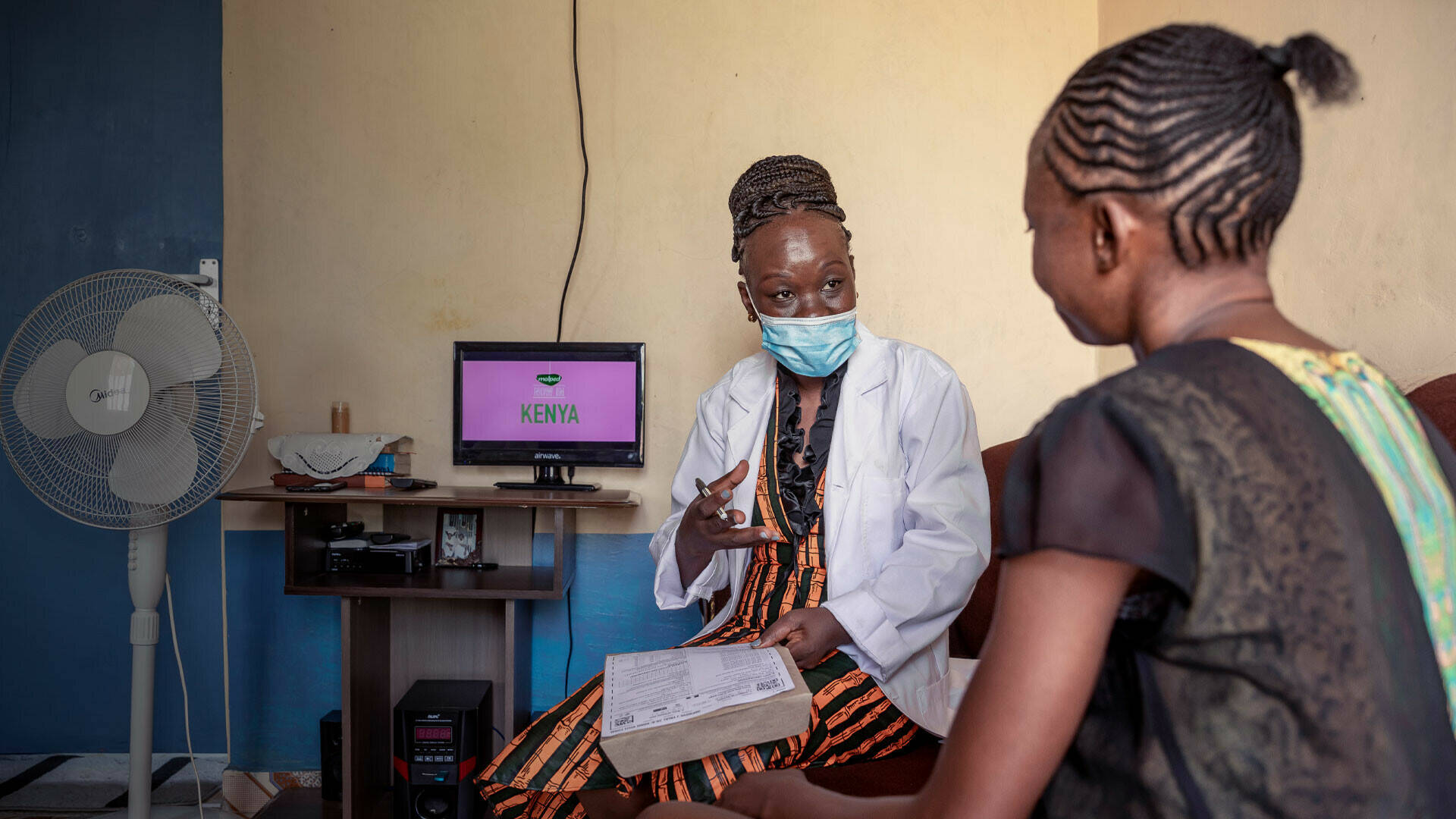
Despite substantial investment in the development of new diagnostics, vaccines and drugs for poverty-related diseases, these interventions may not reach their target populations or be used to their full potential. This is particularly the case in sub-Saharan Africa, where health systems are generally not optimal nor adequately prepared for the delivery and uptake of new or improved products and interventions. Therefore, EDCTP is very intentional in promoting translation of policy-relevant research results into policy and practice.
scroll down
Unnecessary delay or failure to translate research findings into policy and practice prevents research from achieving maximum public health benefit. Therefore, EDCTP is very intentional in promoting translation of policy-relevant research results into policy and practice.
scroll down
Impact of EDCTP-supported activities on policy and practice



Celebrating 20 years of impact through equitable partnerships
The European & Developing Countries Clinical Trials Partnership was established in 2003. This year, we will be celebrating 20 years of supporting equitable European-African research collaborations that have been, and still are, developing new and improved interventions against infectious diseases in sub-Saharan Africa. We invite you to join our Eleventh EDCTP Forum in Paris, France on 7-10 November 2023 and celebrate this momentous occasion with us.
Read the blog
EDCTP: A 20-year track record of supporting excellent, equitable research in Africa (23 June 2022, PLOS Global Public Health Global Health)
Case study 4
CHAPAS trials: Treatments for children with HIV
Case study 3
MVPE-CC: Ensuring malaria vaccine safety and effectiveness
Case study 2
PREPARE and periCOVID Africa: Vaccinating mothers to protect newborns and generating data on COVID-19 infections in pregnant women and their offspring
Case study 1
AMBITION-cm: Simpler and safer treatment of cryptococcal meningitis
Case studies
As part of the 8th edition of the Science Summit around the 77th United Nations General Assembly (UNGA77), EDCTP organised the session ‘Funding research for impact: strengthening the role of science and evidence-based policymaking’. During this session, case studies were presented on advancing the production and usage of reliable research evidence in addressing unmet medical needs, especially amongst vulnerable populations in Africa.
EDCTP investments in R&D

In 435 projects awarded to date.
€814.45 M
Total funding
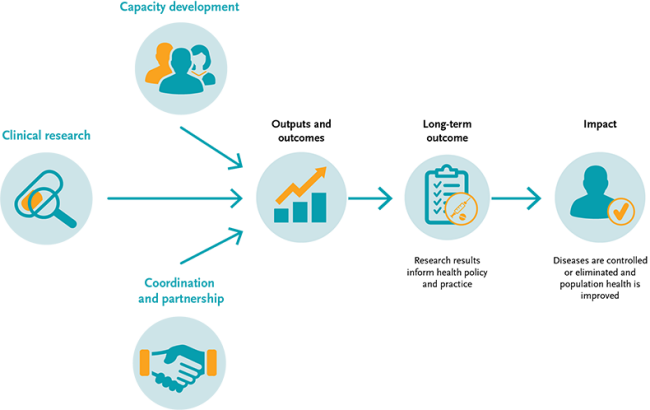
Monitoring & evaluation
In line with internationally recognised principles of results-based management, we monitor and evaluate the EDCTP2 programme to report on its operational performance and results. Our monitoring and evaluation approach draws on a ‘theory of change’ model (see figure below) that maps out the route through which our funding generates immediate outputs (such as the published results from clinical trials), outcomes (such as changes in health policy and practice) and impact (improvements in health and wellbeing and economic gain). This approach helps collect and analyse information from our grants and other activities that are of strategic importance, and more importantly to justify good value-for-money investments.



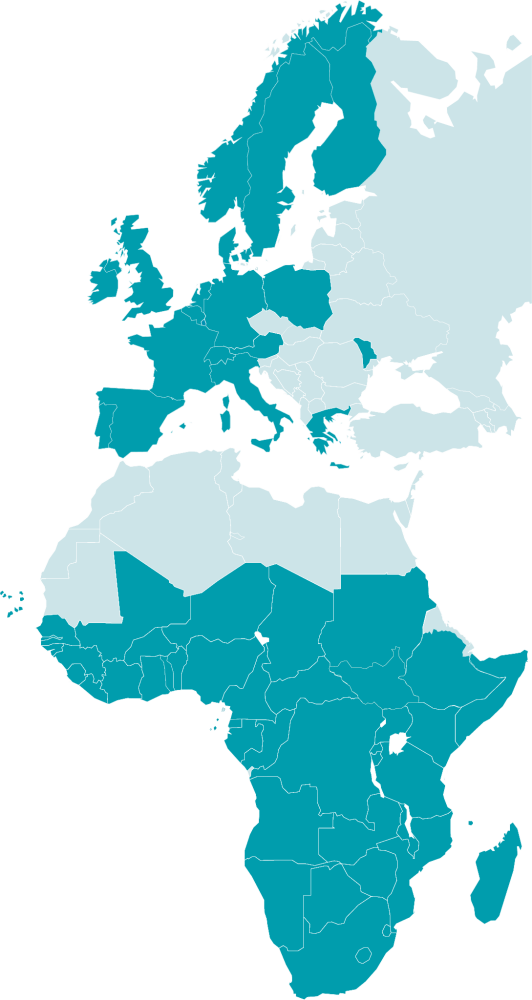
19 European countries
and 44 African countries
63 countries participate in EDCTP-funded activities:
Participation in EDCTP activities
(indicative amounts based on signed grant agreements and proposals approved for funding)
Cumulative investment to call for proposals by year
Investment to call for proposals by year
Investments in calls for proposals
€155.63 M
€16.85 M
€83.42 M
€112.14 M
€814.45 M
€100.27 M
€255.90 M
€444.04 M
€608.47 M
€720.37 M
€94.08 M
2014
2015
2016
2017
2018
2019
2020
€164.19 M
€188.14 M

Facts & figures
By May 2022, the second EDCTP programme (EDCTP2; 2014-2024) portfolio comprised 435 grants awarded through 60 calls for proposals, representing a total investment of EUR 814 million. Clinical trials supported by EDCTP2 involve international collaborations spanning >60 countries and 350 institutions in Europe and sub-Saharan Africa, with broader global collaboration. Results from these clinical trials have generated pivotal evidence which has informed national and international policy and practice.
Despite substantial investment in the development of new diagnostics, vaccines and drugs for poverty-related diseases, these interventions may not reach their target populations or be used to their full potential. This is particularly the case in sub-Saharan Africa, where health systems are generally not optimal nor adequately prepared for the delivery and uptake of new or improved products and interventions. Therefore, EDCTP is very intentional in promoting translation of policy-relevant research results into policy and practice.