CAPA-CT 2
Coordinator: Dr Muhammed Lamorde, IDI, Uganda
Other Beneficiaries:
• University of Turin, Italy
• University of Liverpool, United Kingdom
The 2018/19 Ebola outbreak in the Democratic Republic of the Congo (DRC) has been concentrated in the north-east of the country, an area that shares a border with Uganda. With an estimated 8–10,000 people crossing the border on market days, there is a significant risk that an outbreak could spread from DRC to Uganda. The country has therefore implemented a range of preparedness measures since 2018.
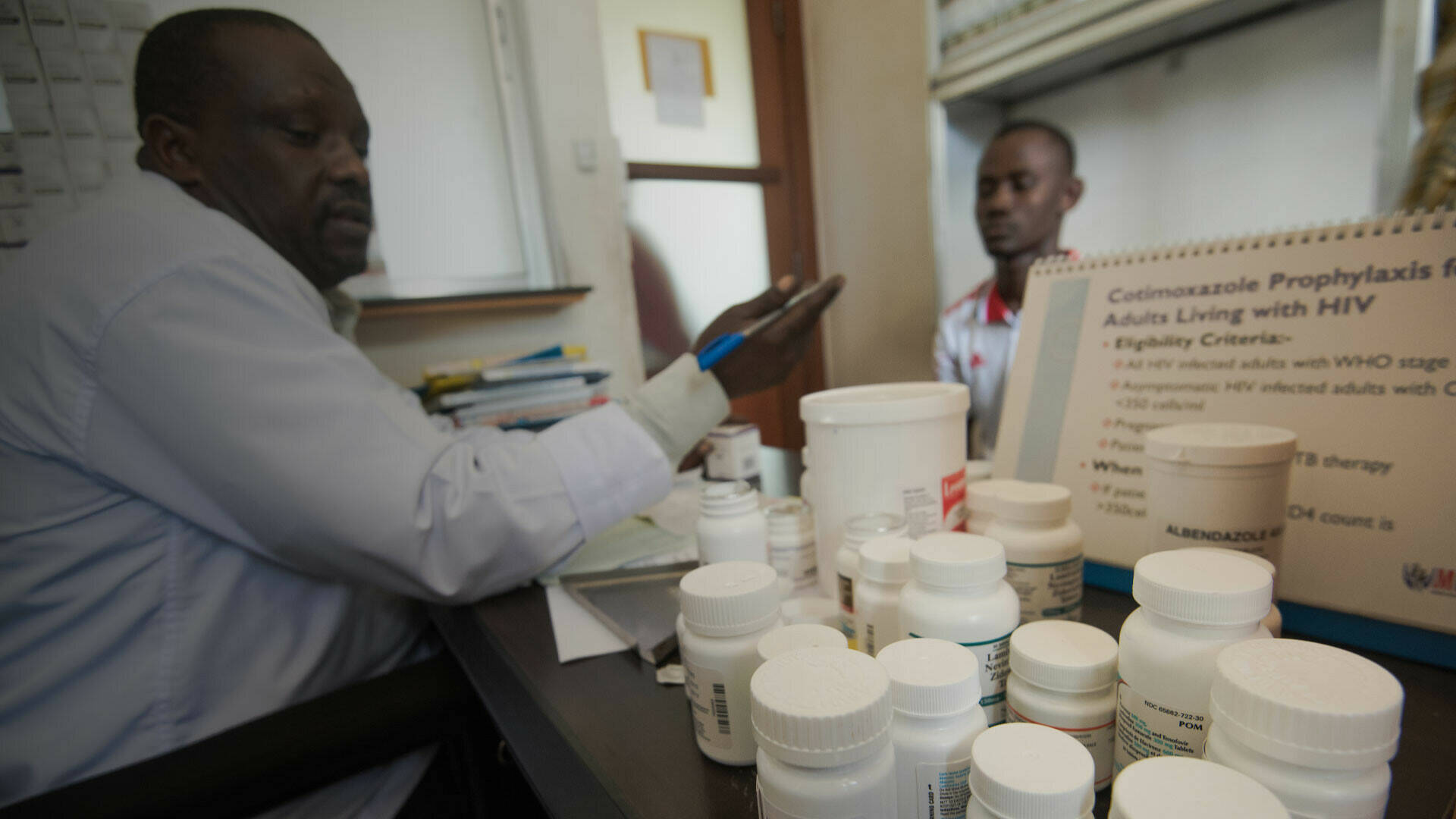
The Infectious Diseases Institute at Makerere University College of Health Sciences in Uganda is a well-established centre of excellence in infectious disease research. The Capa-CT project has built on this foundation, enhancing its capacity to undertake early-phase clinical studies.
To support skills development, the project organised four training workshops on key aspects of clinical management, pharmacokinetic modelling and statistical methods for drug development, delivered to more than 100 local staff. Training was supported by a professor-in-residence initiative. Individual staff received postgraduate training in pharmaceutical medicine and regulatory affairs. One staff member also secured a one-year clinical trial unit placement through a separate EDCTP career development award.
The project also supported upgrading of research facilities and the Institute’s research pharmacies. The Institute also identified potential intensive care unit referral sites and suitable patient transfer arrangements. In addition, the project introduced a range of new approaches to build quality management capacities, and catalysed the creation of a new position, a clinical trials quality manager.
The Capa-CT project built the capacity of Makerere University College of Health Sciences in Uganda to undertake phase I trials of drugs for infectious disease. The CAPA-CT 2 project carried out multiple activities to support Ebola preparedness in Uganda, building on the capacity developed by other EDCTP funding (the CAPA-CT and VirTUAL projects and the EDCTP Senior Fellowship awarded to Dr Michael Walimbwa). First, it seeks to improve knowledge on the mechanism of action of a prioritized investigational anti-Ebola drug (remdesivir) by generating local clinical pharmacokinetic data. Second, it seeks to strengthen surveillance for especially dangerous pathogens in Uganda using diagnostic approaches. Lastly, it seeks to not only contribute to the national response but also to develop a low-cost capacity building model for rapid acquisition of competencies for enhanced laboratory safety and infection, prevention and control to improve the management of patients with suspected Ebola infections.
The CAPA-CT 2 project added to the evidence based on a potentially important new drug therapy for Ebola infections, and contributed to national Ebola preparedness and the development of global health security capacity in Uganda.
Ebola and HIV are found predominately in the same regions of the world and countries in sub-Saharan Africa are most affected by both diseases. For Ebola, no approved therapies exist. However, new investigational drugs are being evaluated to understand if they are effective against the Ebola virus. Remdesivir is an anti-Ebola investigational drug for the treatment of Ebola. Little is known about how the blood levels of remdesivir relate to how effective it is in patients with HIV taking antiretroviral therapy. This study [1]explored how commonly utilized ART (tenofovir/lamivudine and atazanavir/ritonavir) affect the drug levels of remdesivir. Results are expected to include characteristics of remdesivir pharmacokinetics[2] from a typical African population in whom clinical use is anticipated.
[1] https://clinicaltrials.gov/ct2/show/NCT04385719
[2] https://pubmed.ncbi.nlm.nih.gov/34814933/
scroll down

scroll down
CAPA-CT 2

The 2018/19 Ebola outbreak in the Democratic Republic of the Congo (DRC) has been concentrated in the north-east of the country, an area that shares a border with Uganda. With an estimated 8–10,000 people crossing the border on market days, there is a significant risk that an outbreak could spread from DRC to Uganda. The country has therefore implemented a range of preparedness measures since 2018.
The Infectious Diseases Institute at Makerere University College of Health Sciences in Uganda is a well-established centre of excellence in infectious disease research. The Capa-CT project has built on this foundation, enhancing its capacity to undertake early-phase clinical studies.
To support skills development, the project organised four training workshops on key aspects of clinical management, pharmacokinetic modelling and statistical methods for drug development, delivered to more than 100 local staff. Training was supported by a professor-in-residence initiative. Individual staff received postgraduate training in pharmaceutical medicine and regulatory affairs. One staff member also secured a one-year clinical trial unit placement through a separate EDCTP career development award.
The project also supported upgrading of research facilities and the Institute’s research pharmacies. The Institute also identified potential intensive care unit referral sites and suitable patient transfer arrangements. In addition, the project introduced a range of new approaches to build quality management capacities, and catalysed the creation of a new position, a clinical trials quality manager.
The Capa-CT project built the capacity of Makerere University College of Health Sciences in Uganda to undertake phase I trials of drugs for infectious disease. The CAPA-CT 2 project carried out multiple activities to support Ebola preparedness in Uganda, building on the capacity developed by other EDCTP funding (the CAPA-CT and VirTUAL projects and the EDCTP Senior Fellowship awarded to Dr Michael Walimbwa). First, it seeks to improve knowledge on the mechanism of action of a prioritized investigational anti-Ebola drug (remdesivir) by generating local clinical pharmacokinetic data. Second, it seeks to strengthen surveillance for especially dangerous pathogens in Uganda using diagnostic approaches. Lastly, it seeks to not only contribute to the national response but also to develop a low-cost capacity building model for rapid acquisition of competencies for enhanced laboratory safety and infection, prevention and control to improve the management of patients with suspected Ebola infections.
The CAPA-CT 2 project added to the evidence based on a potentially important new drug therapy for Ebola infections, and contributed to national Ebola preparedness and the development of global health security capacity in Uganda.
Ebola and HIV are found predominately in the same regions of the world and countries in sub-Saharan Africa are most affected by both diseases. For Ebola, no approved therapies exist. However, new investigational drugs are being evaluated to understand if they are effective against the Ebola virus. Remdesivir is an anti-Ebola investigational drug for the treatment of Ebola. Little is known about how the blood levels of remdesivir relate to how effective it is in patients with HIV taking antiretroviral therapy. This study [1]explored how commonly utilized ART (tenofovir/lamivudine and atazanavir/ritonavir) affect the drug levels of remdesivir. Results are expected to include characteristics of remdesivir pharmacokinetics[2] from a typical African population in whom clinical use is anticipated.
[1] https://clinicaltrials.gov/ct2/show/NCT04385719
[2] https://pubmed.ncbi.nlm.nih.gov/34814933/
Coordinator: Dr Muhammed Lamorde, IDI, Uganda
Other Beneficiaries:
• University of Turin, Italy
• University of Liverpool, United Kingdom