PEAU-EBOV-RDC
Coordinator: Professor Eric Delaporte, Institut Bouisson Bertrand (IBB)
Other beneficiaries:
- Institut National de Recherche Biomédicale (INRB), Democratic Republic of Congo
- Prins Leopold Instituut voor Tropische Geneeskunde (ITM), Belgium
- University of Oxford, United Kingdom
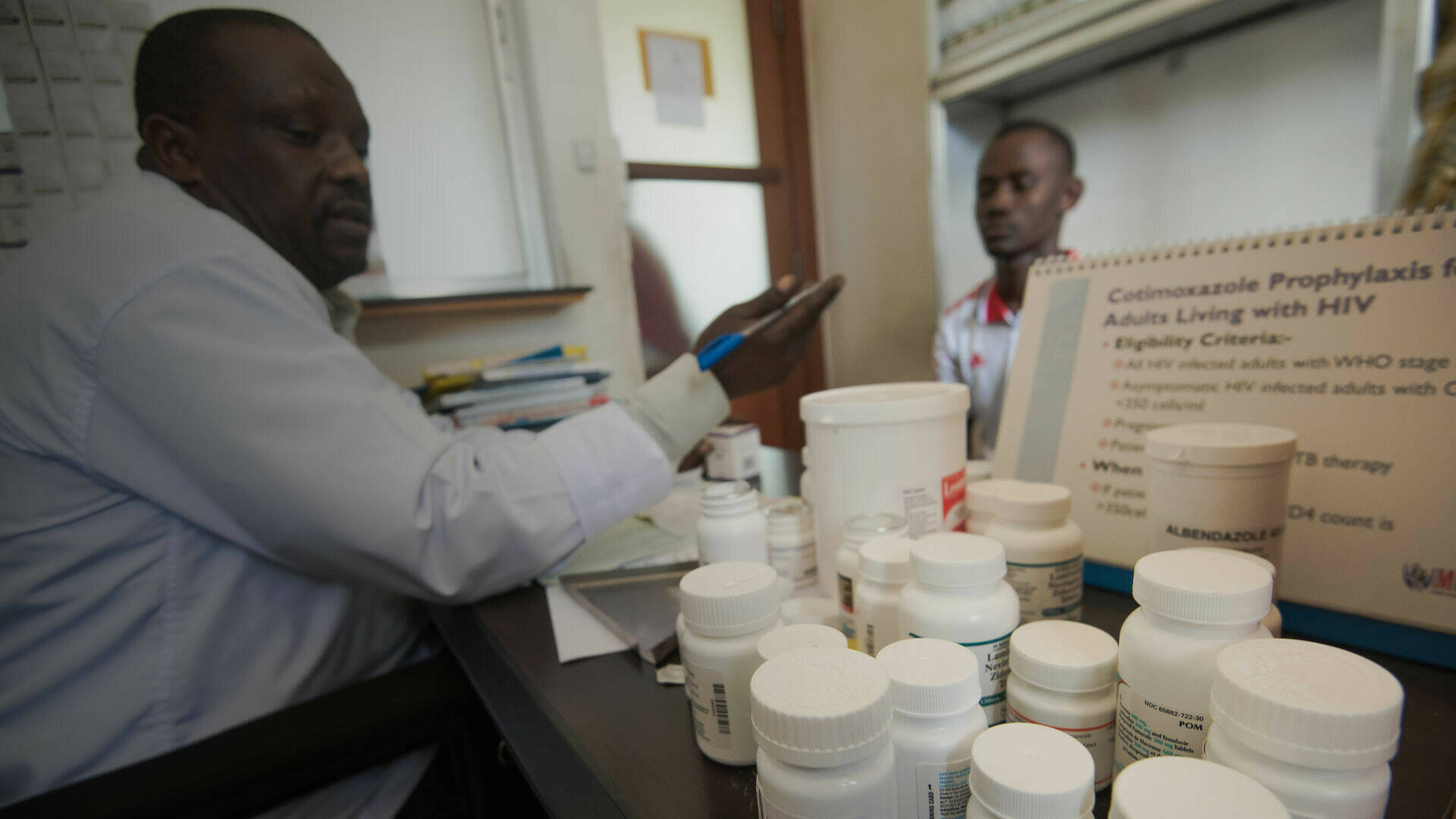
Fatality rates in Ebola outbreaks in the Democratic Republic of the Congo (DRC) have been very high – two-thirds of people with infections have died. There is some evidence that early diagnosis and high-quality patient management can improve survival rates. In addition, a range of new diagnostics and treatments have been developed since the West Africa Ebola outbreak. They may aid survival, but their performance and effectiveness can only be truly tested in outbreak situations. The PEAU-EBOV-RDC study is building the capacity of the Democratic Republic of the Congo to evaluate new diagnostics and treatments for Ebola to reduce very high fatality rates. The project has been developed in partnership with an existing EDCTP-funded network building epidemic preparedness capacities in sub-Saharan Africa, the African Coalition for Epidemic Research, Response and Training (ALERRT).
Initially, the project planned to evaluate new diagnostics and treatments. However, due to a delayed project initiation, as the country went through a period of political unrest, the plans to carry out studies using the MEURI protocol were revised, and the project focused on clinical, immunological of EVD survivors
The PEAU-EBOV-RDC project carried out a range of activities to improve the care of patients with Ebola infections, by enhancing the quality of supportive care. The DRC’s national public health laboratory, l’Institut National de Recherche Biomédicale, has responsibility of coordinating research on Ebola, and had developed a national Ebola research plan. The PEAU-EBOV-RDC project supported a key aim of this plan, through strengthening national capacities to undertake trials, and collect high-quality safety data. It has also supported the strengthening of capacity to handle suspected Ebola samples and diagnose new infections.
Under this grant, the consortium carried out a study[1] aiming to provide a better understanding of Ebola virus infection and its clinical, virological, and immunological consequences, of cured people and their contacts. The results of this study will have a direct impact on the clinical management and on the prevention of possible secondary contamination in the regions with a history of outbreaks.
[1] https://clinicaltrials.gov/ct2/show/NCT04409405
scroll down

scroll down
PEAU-EBOV-RDC

Fatality rates in Ebola outbreaks in the Democratic Republic of the Congo (DRC) have been very high – two-thirds of people with infections have died. There is some evidence that early diagnosis and high-quality patient management can improve survival rates. In addition, a range of new diagnostics and treatments have been developed since the West Africa Ebola outbreak. They may aid survival, but their performance and effectiveness can only be truly tested in outbreak situations. The PEAU-EBOV-RDC study is building the capacity of the Democratic Republic of the Congo to evaluate new diagnostics and treatments for Ebola to reduce very high fatality rates. The project has been developed in partnership with an existing EDCTP-funded network building epidemic preparedness capacities in sub-Saharan Africa, the African Coalition for Epidemic Research, Response and Training (ALERRT).
Initially, the project planned to evaluate new diagnostics and treatments. However, due to a delayed project initiation, as the country went through a period of political unrest, the plans to carry out studies using the MEURI protocol were revised, and the project focused on clinical, immunological of EVD survivors
The PEAU-EBOV-RDC project carried out a range of activities to improve the care of patients with Ebola infections, by enhancing the quality of supportive care. The DRC’s national public health laboratory, l’Institut National de Recherche Biomédicale, has responsibility of coordinating research on Ebola, and had developed a national Ebola research plan. The PEAU-EBOV-RDC project supported a key aim of this plan, through strengthening national capacities to undertake trials, and collect high-quality safety data. It has also supported the strengthening of capacity to handle suspected Ebola samples and diagnose new infections.
Under this grant, the consortium carried out a study[1] aiming to provide a better understanding of Ebola virus infection and its clinical, virological, and immunological consequences, of cured people and their contacts. The results of this study will have a direct impact on the clinical management and on the prevention of possible secondary contamination in the regions with a history of outbreaks.
[1] https://clinicaltrials.gov/ct2/show/NCT04409405
Coordinator: Professor Eric Delaporte, Institut Bouisson Bertrand (IBB)
Other beneficiaries:
- Institut National de Recherche Biomédicale (INRB), Democratic Republic of Congo
- Prins Leopold Instituut voor Tropische Geneeskunde (ITM), Belgium
- University of Oxford, United Kingdom