Tuberculosis (TB) kills more people than any other single microorganism. Over the last two decades, EDCTP has made significant research investments in all types of innovative medical interventions to end TB. EDCTP’s portfolio on TB research includes several major vaccine development projects, projects to develop new drug regimens to shorten the duration of treatment, and projects on innovative diagnostics.


We asked Prof. Chegou how his EDCTP Fellowship has helped him to establish himself as a research leader through additional technical training, mentoring and leadership skills development.

Interview:
Prof. Novel Chegou


The TriageTB project is led by the AE-TBC Consortium which won the EDCTP Outstanding Research Team Prize 2020.

The TB-CAPT CORE study is generating evidence on a new TB diagnostic and resistance-profiling test, Truenat MTB Plus, which was endorsed by WHO in 2020. As it is battery-powered and portable, Truenat technology has the key advantage that it can be used at peripheral primary health care facilities. The trial is comparing the use of Truenat with standard of care. The trial completed recruitment including3987 patients at 28 primary health care facilities in Tanzania and Mozambique.
The third study, TB-CAPT EXULTANT HIV, is evaluating the impact of an expanded testing strategy with Ultra on GeneXpert for diagnosing TB in people living with HIV admitted to hospital, who often have undiagnosed TB. The study investigates the use of alternative sample sources, such as urine or stool, and diagnostic technologies such as the urine-based AlereLAM assay. The study completed recruitment including 1172 participants from Mozambique and Tanzania.
Results from the TB-CAPT CORE and TB-CAPT EXULTANT HIV studies are expected to be published in early 2025.
The EDCTP2-funded Cough Audio triaGE for TB (CAGE-TB) project is developing an innovative, inexpensive cough audio classifier to identify missing (undiagnosed) TB cases. Using a smartphone with the cough classifier app, healthworkers would test people entering the health clinic by analysing the sound of their cough. On the basis of the cough analysis, only suspected TB cases would proceed to follow-up diagnosis. The use of this innovative, simple and cheap technology could enable more missing TB cases to be identified while saving resources. Furthermore, by implementing the classifier through a user-friendly smartphone app, the tool would be relatively easy to implement at health facilities in high-burden areas.
Global Health EDCTP3 programme is now providing further funding to support the continuation, and successful delivery of this project, under the new name of 4-CAGE-TB, after disruption of the original project due to COVID-19. The 4-CAGE-TB project will complete wider testing of the cough analyser in Uganda and South Africa. Additionally, consultations with patients, health workers and national TB programmes will be conducted to understand how best to ensure acceptability, uptake and implementation, and raise awareness of the product in local communities.
The project is led by Professor Grant Theron (Stellenbosch University, South Africa), a former EDCTP Senior Fellow and Career Development Fellow, and an emerging authority on TB diagnostics. He was part of the team that demonstrated the practicability of the Xpert molecular diagnostic platform, informing WHO recommendations on its use. He also played a key role in highly influential EDCTP1-funded studies revealing that, in practice, Xpert had less impact than expected because clinicians’ treatment choices were only partly based on diagnostic results – emphasising the importance of understanding the context in which diagnostic tests are used.
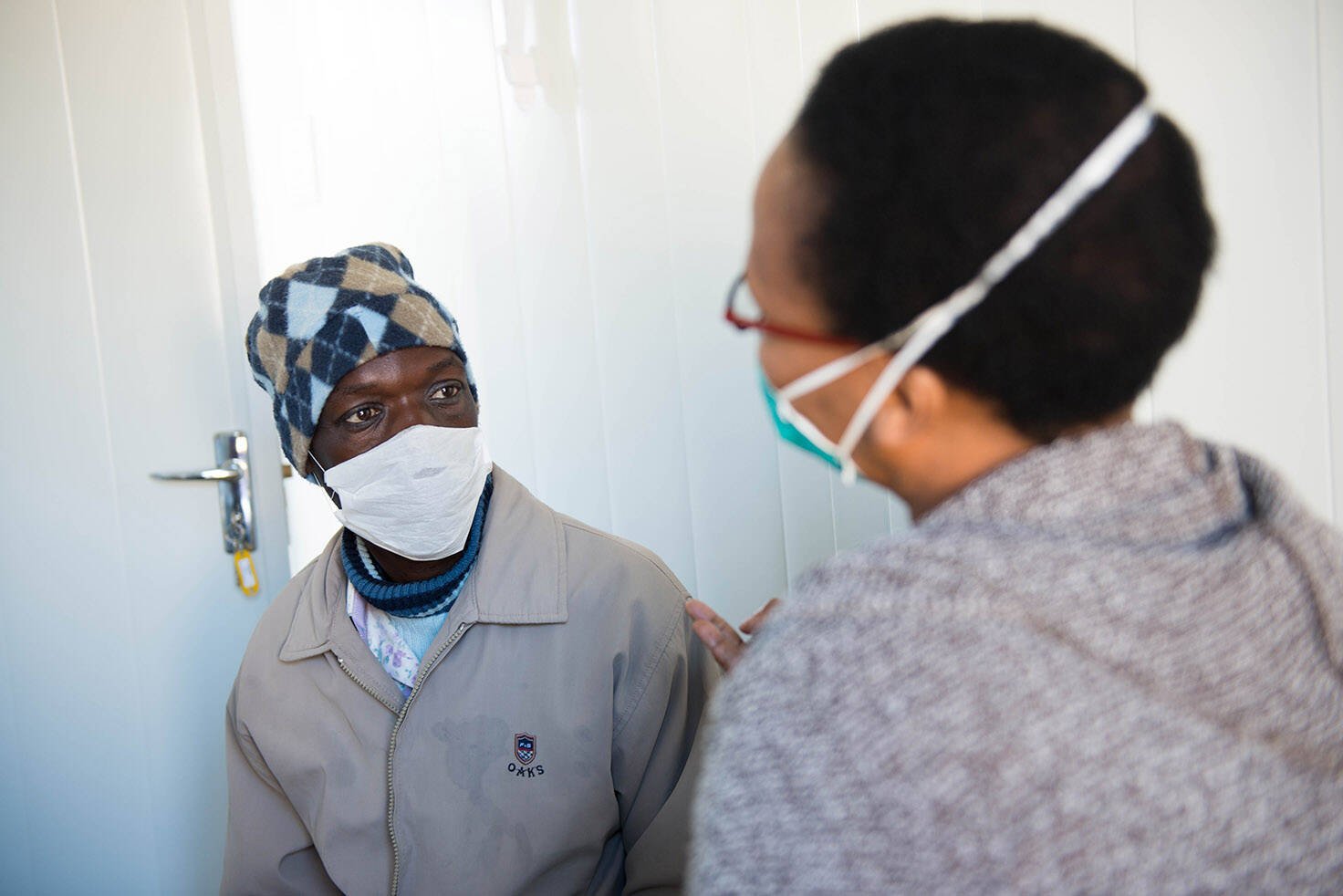
Research capacity strengthening is a major component of the TriageTB project. The activities included a mentorship program, training in clinical and laboratory methods of African scientists and formal post-graduate training for masters and doctoral studies. The EDCTP Senior Fellow Professor Novel Chegou (Stellenbosch University, South Africa), is the Project Scientist of the TriageTB site in South Africa. He was also part of previous EDCTP-funded projects, AE-TBC and ScreenTB. The AE-TBC project identified a set of biomarkers associated with TB meningitis, a potentially fatal mycobacterial infection of brain tissue. The ScreenTB project evaluated the potential of a point-of-care diagnostic based on these biosignatures to detect active TB.
In his Senior Fellowship project, Professor Chegou is working with colleagues in the Engineering Department of Stellenbosch University to develop a biomarker-based point-of-care test for TB meningitis in children, the most severe form of the disease. Currently diagnosis relies on imaging or analysis of cerebrospinal fluid, which requires specialist facilities. Many children die or suffer life-saving disabilities because of this delay in diagnosis.
Considering the importance of his work, in 2022 the UK’s Royal Society awarded Professor Chegou its Royal Society Africa Prize, which recognises the innovative contribution of an African research scientist.
Timely TB diagnosis remains a major challenge. Although accurate and reliable molecular testing platforms now exist, tests are too expensive to be used on all suspected cases. A high development priority is therefore ‘triage’ tests that can efficiently detect likely TB cases for confirmatory molecular testing.
The TriageTB project team has been searching for host biomarkers that could act as signatures of active TB infections, with the aim of developing a cheap, easy-to-use dipstick test to be used as a point-of-care diagnostic
TriageTB evaluated the use of a simple point-of-care fingerprick test to detect active TB infections in symptomatic patients. Previous EDCTP-funded projects have identified markers in blood that can act as a distinctive signature of TB infection. The TriageTB project was aligned with the ENDxTB study, funded by the US NIH to increase global coverage, has used samples from sites around the world to refine the test so it could be used globally. In Triage TB, the test was evaluated in The Gambia, South Africa and Uganda, with 909 participants recruited.
Ultimately, the test could have most value as a non-sputum based ‘rule out’ test, with a negative test suggesting that TB infection is highly unlikely, and so only those testing positive would undergo molecular testing. Initial results suggest that this could cut the number of expensive negative molecular tests by around 75%. The results from the TriageTB project will be published in 2025.


Molecular testing for TB offered the prospect of rapid detection of infection and timely initiation of treatment. However, its impact has not been as great as expected, highlighting the need to consider practical issues of how molecular testing fits into clinical decision-making and working practices in existing healthcare systems in sub-Saharan Africa.
The TB CAPT project is performing three clinical studies focused on optimising the use of different molecular testing tools in sub-Saharan African settings. Its TB CAPT XDR study is exploring the feasibility and impact of the WHO-approved Xpert MTB/XDR test when integrated within the existing TB diagnostics infrastructure in South Africa. The study showed the efficacy of the Xpert MTB/XDR test in detecting Mycobacterium tuberculosis and in testing for resistance to crucial drugs on residual sputum already processed for Xpert MTB/RIF Ultra. The study highlights that the Xpert MTB/XDR test provided comprehensive resistance results in 84% of cases compared to 66% with routine testing, significantly reducing the time to get results from 15 days to just 23 hours. This approach of making use of residual specimen not only increases the availability of diagnostic results but also accelerates the diagnostic process, making it a pivotal advancement in the fight against tuberculosis.
scroll down
TB is difficult to diagnose on the basis of clinical symptoms. Culture methods take weeks to deliver results, and molecular diagnostics are expensive and often require specialist facilities. As a result, there is an urgent need for novel tools and testing practices to efficiently and more rapidly detect TB cases so that treatment can be started promptly – benefiting patients and reducing the risk of further disease transmission.
In response to that, EDCTP has supported multiple studies evaluating different strategies and innovative technologies for better and more rapid identification of TB cases.
EDCTP2's investment in tuberculosis research by intervention
€8.55 M
Between 2014 and 2024, EDCTP has invested € 213.50 million in collaborative clinical studies on TB, capacity building and talent development through its second programme (EDCTP2), featuring as the second largest public funder of TB research and the third largest funder of TB research overall in the 2021 Report on TB Research Funding Trends, a publication of the Treatment Action Group. Of the total investment in TB, almost 40% (€84.70 million) is to support clinical research and capacity building activities that focus on TB diagnostics.

Tuberculosis (TB) kills more people than any other single microorganism. Over the last two decades, EDCTP has made significant research investments in all types of innovative medical interventions to end TB. EDCTP’s portfolio on TB research includes several major vaccine development projects, projects to develop new drug regimens to shorten the duration of treatment, and projects on innovative diagnostics.
scroll down

The EDCTP2-funded Cough Audio triaGE for TB (CAGE-TB) project is developing an innovative, inexpensive cough audio classifier to identify missing (undiagnosed) TB cases. Using a smartphone with the cough classifier app, healthworkers would test people entering the health clinic by analysing the sound of their cough. On the basis of the cough analysis, only suspected TB cases would proceed to follow-up diagnosis. The use of this innovative, simple and cheap technology could enable more missing TB cases to be identified while saving resources. Furthermore, by implementing the classifier through a user-friendly smartphone app, the tool would be relatively easy to implement at health facilities in high-burden areas.
Global Health EDCTP3 programme is now providing further funding to support the continuation, and successful delivery of this project, under the new name of 4-CAGE-TB, after disruption of the original project due to COVID-19. The 4-CAGE-TB project will complete wider testing of the cough analyser in Uganda and South Africa. Additionally, consultations with patients, health workers and national TB programmes will be conducted to understand how best to ensure acceptability, uptake and implementation, and raise awareness of the product in local communities.
The project is led by Professor Grant Theron (Stellenbosch University, South Africa), a former EDCTP Senior Fellow and Career Development Fellow, and an emerging authority on TB diagnostics. He was part of the team that demonstrated the practicability of the Xpert molecular diagnostic platform, informing WHO recommendations on its use. He also played a key role in highly influential EDCTP1-funded studies revealing that, in practice, Xpert had less impact than expected because clinicians’ treatment choices were only partly based on diagnostic results – emphasising the importance of understanding the context in which diagnostic tests are used.
The TB-CAPT CORE study is generating evidence on a new TB diagnostic and resistance-profiling test, Truenat MTB Plus, which was endorsed by WHO in 2020. As it is battery-powered and portable, Truenat technology has the key advantage that it can be used at peripheral primary health care facilities. The trial is comparing the use of Truenat with standard of care. The trial completed recruitment including3987 patients at 28 primary health care facilities in Tanzania and Mozambique.
The third study, TB-CAPT EXULTANT HIV, is evaluating the impact of an expanded testing strategy with Ultra on GeneXpert for diagnosing TB in people living with HIV admitted to hospital, who often have undiagnosed TB. The study investigates the use of alternative sample sources, such as urine or stool, and diagnostic technologies such as the urine-based AlereLAM assay. The study completed recruitment including 1172 participants from Mozambique and Tanzania.
Results from the TB-CAPT CORE and TB-CAPT EXULTANT HIV studies are expected to be published in early 2025.
EDCTP2's investment in tuberculosis research by intervention
€8.55 M
Between 2014 and 2024, EDCTP has invested € 213.50 million in collaborative clinical studies on TB, capacity building and talent development through its second programme (EDCTP2), featuring as the second largest public funder of TB research and the third largest funder of TB research overall in the 2021 Report on TB Research Funding Trends, a publication of the Treatment Action Group. Of the total investment in TB, almost 40% (€84.70 million) is to support clinical research and capacity building activities that focus on TB diagnostics.


We asked Prof. Chegou how his EDCTP Fellowship has helped him to establish himself as a research leader through additional technical training, mentoring and leadership skills development.

Interview:
Prof. Novel Chegou
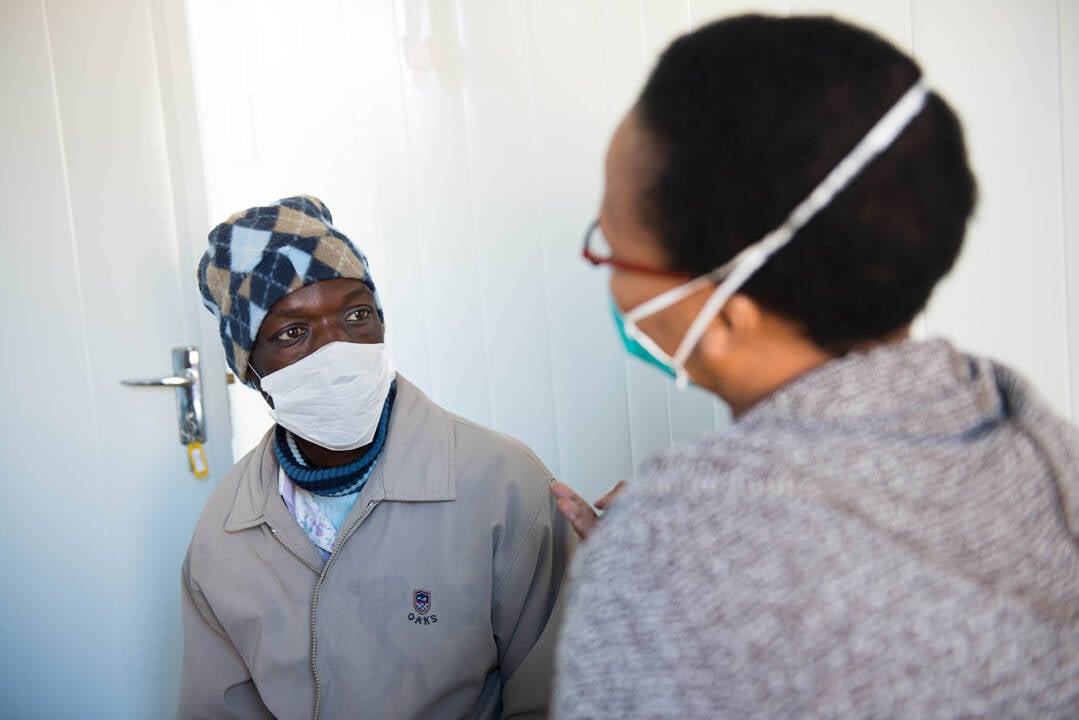

The TriageTB project is led by the AE-TBC Consortium which won the EDCTP Outstanding Research Team Prize 2020.
Timely TB diagnosis remains a major challenge. Although accurate and reliable molecular testing platforms now exist, tests are too expensive to be used on all suspected cases. A high development priority is therefore ‘triage’ tests that can efficiently detect likely TB cases for confirmatory molecular testing.
The TriageTB project team has been searching for host biomarkers that could act as signatures of active TB infections, with the aim of developing a cheap, easy-to-use dipstick test to be used as a point-of-care diagnostic
TriageTB evaluated the use of a simple point-of-care fingerprick test to detect active TB infections in symptomatic patients. Previous EDCTP-funded projects have identified markers in blood that can act as a distinctive signature of TB infection. The TriageTB project was aligned with the ENDxTB study, funded by the US NIH to increase global coverage, has used samples from sites around the world to refine the test so it could be used globally. In Triage TB, the test was evaluated in The Gambia, South Africa and Uganda, with 909 participants recruited.
Ultimately, the test could have most value as a non-sputum based ‘rule out’ test, with a negative test suggesting that TB infection is highly unlikely, and so only those testing positive would undergo molecular testing. Initial results suggest that this could cut the number of expensive negative molecular tests by around 75%. The results from the TriageTB project will be published in 2025.
Molecular testing for TB offered the prospect of rapid detection of infection and timely initiation of treatment. However, its impact has not been as great as expected, highlighting the need to consider practical issues of how molecular testing fits into clinical decision-making and working practices in existing healthcare systems in sub-Saharan Africa.
The TB CAPT project is performing three clinical studies focused on optimising the use of different molecular testing tools in sub-Saharan African settings. Its TB CAPT XDR study is exploring the feasibility and impact of the WHO-approved Xpert MTB/XDR test when integrated within the existing TB diagnostics infrastructure in South Africa. The study showed the efficacy of the Xpert MTB/XDR test in detecting Mycobacterium tuberculosis and in testing for resistance to crucial drugs on residual sputum already processed for Xpert MTB/RIF Ultra. The study highlights that the Xpert MTB/XDR test provided comprehensive resistance results in 84% of cases compared to 66% with routine testing, significantly reducing the time to get results from 15 days to just 23 hours. This approach of making use of residual specimen not only increases the availability of diagnostic results but also accelerates the diagnostic process, making it a pivotal advancement in the fight against tuberculosis.

TB is difficult to diagnose on the basis of clinical symptoms. Culture methods take weeks to deliver results, and molecular diagnostics are expensive and often require specialist facilities. As a result, there is an urgent need for novel tools and testing practices to efficiently and more rapidly detect TB cases so that treatment can be started promptly – benefiting patients and reducing the risk of further disease transmission.
In response to that, EDCTP has supported multiple studies evaluating different strategies and innovative technologies for better and more rapid identification of TB cases.