
Michelle Helinski, Ana Lúcia Weinberg, Ian Jones

Tuberculosis (TB) is a major global public health challenge, resulting in more than 1.4 million deaths a year. Although ambitious global TB control goals have been established, it is widely recognised that these will not be achieved without new vaccines. However, there is little commercial incentive to develop new TB vaccines as TB is a disease of poverty and plagues primarily low- and middle-income countries. Despite the huge global burden of disease, no new vaccine has been introduced since BCG in the 1920s.
A Global TB Vaccine
R&D Roadmap
Addressing this need will require cross-sectoral partnerships and coordinated “end-to-end” development, from early-stage discovery research through to programmatic implementation. As a first step, the Global TB Vaccine R&D Roadmap has been developed through an iterative global consultative process to identify the key barriers and the ways in which they might be overcome. The roadmap is intended to provide a shared set of priorities to guide the activities of all stakeholders with an interest in TB vaccine development and use.

A key milestone in new product development is licensing approval, but this does not by itself deliver public health benefit. Multiple barriers may hamper the timely implementation of a newly licensed TB vaccine. Key challenges include limited understanding of how a new vaccine would be used within different countries, as well as the lack of investment cases to support national and global decision-making on procurement and implementation. To address these issues there is a need to:
- Collect data to model potential impacts of vaccine use and develop country-use scenarios and investment cases
- Undertake activities to prepare countries for evaluation of newly licensed vaccines and rapid implementation once recommended locally.
- Identify and develop strategies to address potential barriers to implementation, including community resistance to TB vaccination.
- Post-licensure studies should be planned to establish vaccine effectiveness.
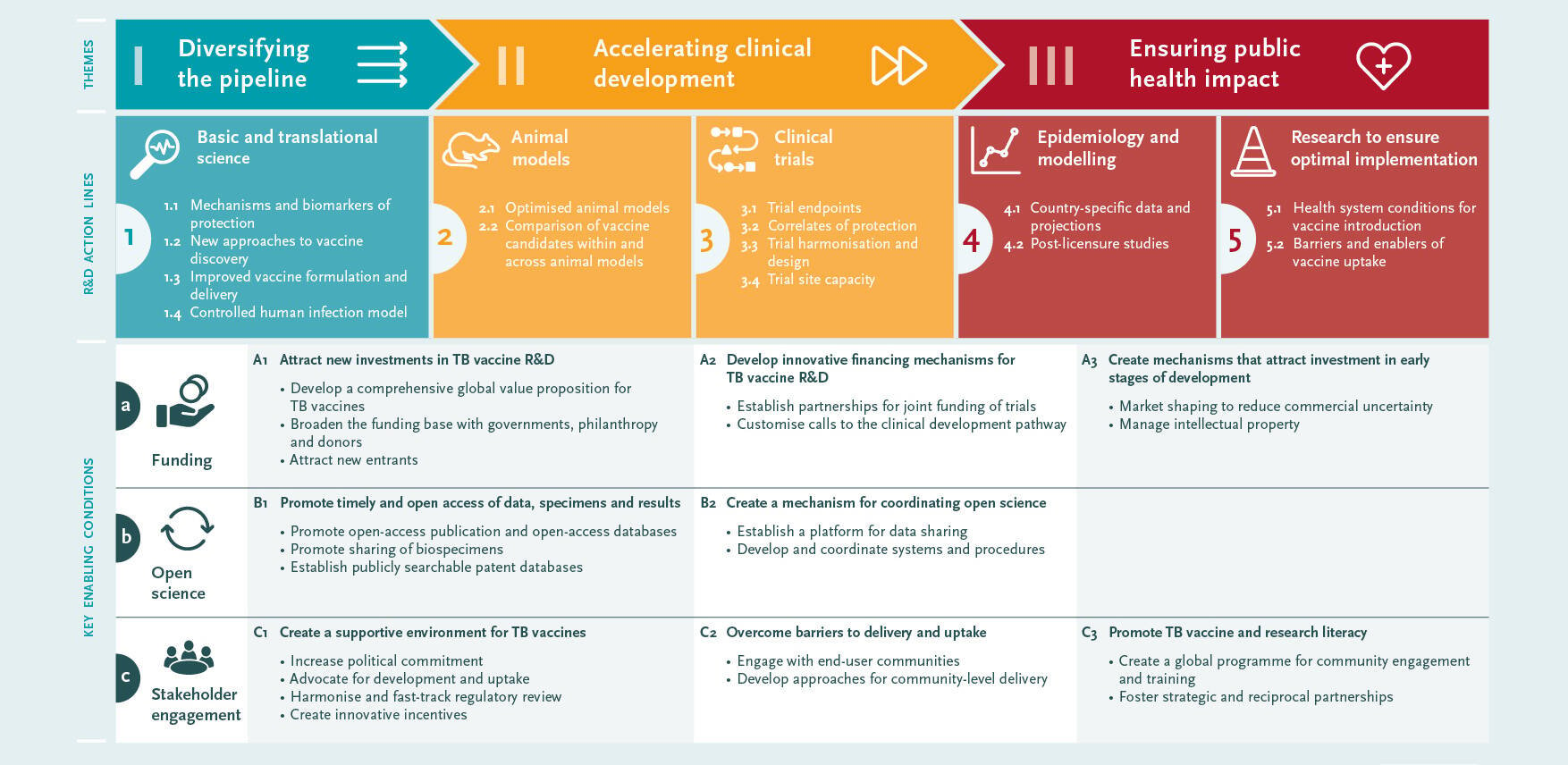
The Roadmap consultation identified three priority areas:1) diversifying the pipeline, 2) accelerating clinical development, and 3) ensuring public health impact.
The TB vaccine pipeline is not well-stocked and lacks diversity. Early-stage R&D has focused on a limited number of Mycobacterium tuberculosis (Mtb) antigens and on a very specific type of immune response. There is a need to:
- Increase the diversity of the pipeline by focusing on a wider range of antigens, immune responses and delivery mechanisms.
- Improve understanding of correlates of protection and identify biosignatures that correlate with vaccine-induced protection.
- Improve understanding of mucosal immune responses in the lung and how they relate to systemic responses.
- Explore the potential use of controlled human infection models.
Clinical evaluation of TB vaccines is slow, costly and high-risk. In part, this reflects the fact that efficacy in animal models is not a good predictor of efficacy in people, making it difficult to prioritise candidates for clinical evaluation. A further important barrier is the lack of well-defined correlates of protection or proxy measures of efficacy. Demonstration of efficacy therefore requires large trials with prevention of disease as the primary endpoint. There is a need to:
- Optimise animal models by “back-translating” findings from successful clinical trials.
- Optimise alternative endpoints, by establishing the relationship between prevention of disease and other possible endpoints (particularly prevention of infection and prevention of recurrence).
- Harmonising and standardising trial protocols, and exploring innovative trial designs to improve efficiency.
- Last but not least, clinical trial capacity must be built in high-burden countries.
Priorities
Dr Michael Makanga, EDCTP Executive Director: “The main drive for investing in this very consultative new global TB vaccine roadmap is our enduring commitment to a concerted global R&D effort that will ultimately end TB. This timely Roadmap may leverage recent advances and new opportunities arising from lessons learnt from other diseases such as COVID-19”.
scroll down

Three cross-cutting enabling factors were also identified: 1) funding; 2) open science, and 3) stakeholder engagement.
TB research is underfunded globally and TB vaccine research is particularly poorly resourced. Interest in funding TB vaccine R&D needs to be increased and more funders need to be involved. Greater coordination across funders could help to increase R&D productivity and accelerate clinical evaluation. To create greater commercial interest in TB vaccine R&D, innovative funding (“push”) mechanisms need to be combined with market-shaping (“pull”) approaches, such as advance market commitments.
Global efforts are required to encourage ‘open science’ with greater sharing of data and specimens, facilitated by the establishment of biobanks and data platforms. A failure to publish data, particularly negative results, can lead to unnecessary duplication of efforts and research being carried out on approaches unlikely to be successful.
A wide range of stakeholders needs to be engaged, including global agencies, industry, regulatory authorities, national decision-makers and local communities. Additional investment in TB vaccine R&D, awareness of the need for and possibilities of new TB vaccines, and community support for vaccination are the main goals of this engagement.
Enabling factors
One hundred years after the BCG vaccine, there is real hope that disadvantaged populations around the world can finally gain the benefits of TB prevention and cure through the use of vaccination.
New TB vaccines will fulfil multiple roles in TB control but a stronger and more diverse TB vaccine pipeline needs to be build. It is equally important to begin preparing the ground for the introduction of new TB vaccines. An end-to-end perspective needs to be adopted. Clinical development must be informed by a sound understanding of national decision-making and implementation constraints, while licensing must be swiftly followed by efficient programmatic implementation.
If all stakeholders collectively and collaboratively focus on the priorities identified in this Roadmap, progress will be accelerated. The unprecedented speed at which COVID-19 vaccines have been developed holds important lessons that can be applied to TB vaccines.
The road to End TB
The Roadmap and supporting documents can be found via the EDCTP news item.
EDCTP and AIGHD launched a global roadmap for tuberculosis vaccine development (20 April 2021)
and also under EDCTP Publications:
Global roadmap for research and development of tuberculosis vaccines (20 April 2021)
Related news items:
EDCTP-AIGHD Special Session at the Virtual Global Forum on TB Vaccines: Launch of a Global Roadmap (20 April 2021)
EDCTP - Public consultation: Global Roadmap for research and development for TB vaccines (13 January 2021)
AIGHD - EDCTP asks AIGHD to develop road map for tuberculosis vaccines (28 August 2020)
EDCTP - Developing a Global TB Vaccine R&D Roadmap
(02 March 2020)
More information

Michelle Helinski, Ana Lúcia Weinberg, Ian Jones
A Global TB Vaccine
R&D Roadmap
scroll down
Tuberculosis (TB) is a major global public health challenge, resulting in more than 1.4 million deaths a year. Although ambitious global TB control goals have been established, it is widely recognised that these will not be achieved without new vaccines. However, there is little commercial incentive to develop new TB vaccines as TB is a disease of poverty and plagues primarily low- and middle-income countries. Despite the huge global burden of disease, no new vaccine has been introduced since BCG in the 1920s.
Addressing this need will require cross-sectoral partnerships and coordinated “end-to-end” development, from early-stage discovery research through to programmatic implementation. As a first step, the Global TB Vaccine R&D Roadmap has been developed through an iterative global consultative process to identify the key barriers and the ways in which they might be overcome. The roadmap is intended to provide a shared set of priorities to guide the activities of all stakeholders with an interest in TB vaccine development and use.
Dr Michael Makanga, EDCTP Executive Director: “The main drive for investing in this very consultative new global TB vaccine roadmap is our enduring commitment to a concerted global R&D effort that will ultimately end TB. This timely Roadmap may leverage recent advances and new opportunities arising from lessons learnt from other diseases such as COVID-19”.
A key milestone in new product development is licensing approval, but this does not by itself deliver public health benefit. Multiple barriers may hamper the timely implementation of a newly licensed TB vaccine. Key challenges include limited understanding of how a new vaccine would be used within different countries, as well as the lack of investment cases to support national and global decision-making on procurement and implementation. To address these issues there is a need to:
- Collect data to model potential impacts of vaccine use and develop country-use scenarios and investment cases
- Undertake activities to prepare countries for evaluation of newly licensed vaccines and rapid implementation once recommended locally.
- Identify and develop strategies to address potential barriers to implementation, including community resistance to TB vaccination.
- Post-licensure studies should be planned to establish vaccine effectiveness.
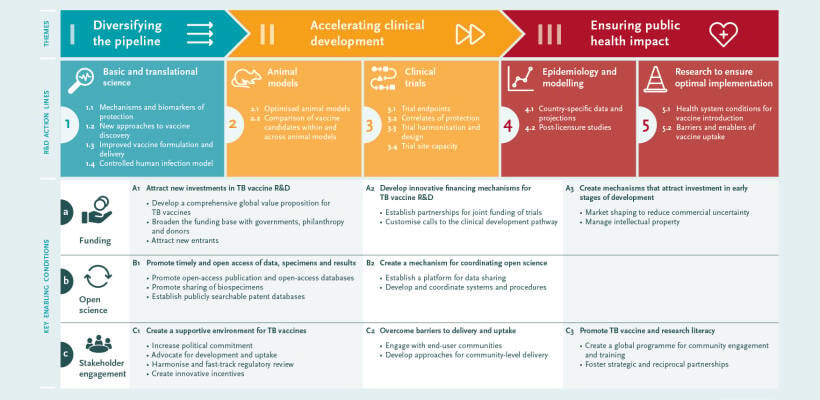
The Roadmap consultation identified three priority areas:1) diversifying the pipeline, 2) accelerating clinical development, and 3) ensuring public health impact.
The TB vaccine pipeline is not well-stocked and lacks diversity. Early-stage R&D has focused on a limited number of Mycobacterium tuberculosis (Mtb) antigens and on a very specific type of immune response. There is a need to:
- Increase the diversity of the pipeline by focusing on a wider range of antigens, immune responses and delivery mechanisms.
- Improve understanding of correlates of protection and identify biosignatures that correlate with vaccine-induced protection.
- Improve understanding of mucosal immune responses in the lung and how they relate to systemic responses.
- Explore the potential use of controlled human infection models.
Clinical evaluation of TB vaccines is slow, costly and high-risk. In part, this reflects the fact that efficacy in animal models is not a good predictor of efficacy in people, making it difficult to prioritise candidates for clinical evaluation. A further important barrier is the lack of well-defined correlates of protection or proxy measures of efficacy. Demonstration of efficacy therefore requires large trials with prevention of disease as the primary endpoint. There is a need to:
- Optimise animal models by “back-translating” findings from successful clinical trials.
- Optimise alternative endpoints, by establishing the relationship between prevention of disease and other possible endpoints (particularly prevention of infection and prevention of recurrence).
- Harmonising and standardising trial protocols, and exploring innovative trial designs to improve efficiency.
- Last but not least, clinical trial capacity must be built in high-burden countries.
Priorities


Three cross-cutting enabling factors were also identified: 1) funding; 2) open science, and 3) stakeholder engagement.
TB research is underfunded globally and TB vaccine research is particularly poorly resourced. Interest in funding TB vaccine R&D needs to be increased and more funders need to be involved. Greater coordination across funders could help to increase R&D productivity and accelerate clinical evaluation. To create greater commercial interest in TB vaccine R&D, innovative funding (“push”) mechanisms need to be combined with market-shaping (“pull”) approaches, such as advance market commitments.
Global efforts are required to encourage ‘open science’ with greater sharing of data and specimens, facilitated by the establishment of biobanks and data platforms. A failure to publish data, particularly negative results, can lead to unnecessary duplication of efforts and research being carried out on approaches unlikely to be successful.
A wide range of stakeholders needs to be engaged, including global agencies, industry, regulatory authorities, national decision-makers and local communities. Additional investment in TB vaccine R&D, awareness of the need for and possibilities of new TB vaccines, and community support for vaccination are the main goals of this engagement.
Enabling factors
One hundred years after the BCG vaccine, there is real hope that disadvantaged populations around the world can finally gain the benefits of TB prevention and cure through the use of vaccination.
New TB vaccines will fulfil multiple roles in TB control but a stronger and more diverse TB vaccine pipeline needs to be build. It is equally important to begin preparing the ground for the introduction of new TB vaccines. An end-to-end perspective needs to be adopted. Clinical development must be informed by a sound understanding of national decision-making and implementation constraints, while licensing must be swiftly followed by efficient programmatic implementation.
If all stakeholders collectively and collaboratively focus on the priorities identified in this Roadmap, progress will be accelerated. The unprecedented speed at which COVID-19 vaccines have been developed holds important lessons that can be applied to TB vaccines.
The road to End TB
The Roadmap and supporting documents can be found via the EDCTP news item.
EDCTP and AIGHD launched a global roadmap for tuberculosis vaccine development (20 April 2021)
and also under EDCTP Publications:
Global roadmap for research and development of tuberculosis vaccines (20 April 2021)
Related news items:
EDCTP-AIGHD Special Session at the Virtual Global Forum on TB Vaccines: Launch of a Global Roadmap (20 April 2021)
More information
