EDCTP activated this mechanism in April 2020 to support collaborative clinical research studies in sub-Saharan Africa to manage and/or prevent the spread of the COVID-19 epidemic. As of March 2021, twenty-six projects are ongoing in 22 countries across sub-Saharan Africa. One year on from the declaration of COVID-19 as a pandemic, many research questions have been answered, many others are still under investigation and new questions are emerging.
The objectives of the webinar were threefold. First, to share experience and study findings among the EDCTP COVID-19 research consortia. Secondly, to discuss new emerging questions and the current public health priorities in sub-Saharan Africa. Thirdly, to inform the global research community and stakeholders about this EDCTP-funded COVID-19 research.
The webinar was divided in four sessions focused on, respectively:
1) surveillance and response strategies;
2) special or key populations;
3) COVID-19 and other major infectious diseases; and
4) new emerging questions and knowledge gaps specific
to sub-Saharan Africa.
scroll down
The second session included presentations from the periCOVID-Africa, COVAB and COREP consortia, and concluded with a panel discussion where the three presenters were joined by investigators from Profile-Cov and RADIATES consortia.
The key point made in this session was the need to address the knowledge gaps regarding risk, transmission and immunity in pregnant women and their infants. A call was made to increase the inclusion of pregnant women in ongoing and planned clinical studies but also in the planned safety studies that will accompany vaccine roll-out programmes. A lack of vaccines safety data for pregnant women may affect vaccines acceptance.
Speakers also explored the broadening definition of key populations in the context of COVID-19. Patients with non-communicable conditions such as obesity would fall under the definition but also varying categories of front-line workers. Depending on the socio-economic situation in various countries front-line workers could include police agents or bankers. The issue of stigma was mentioned again. Speakers stressed the impact that mistrust and stigma may have on research response activities, by keeping key populations away from studies.
Key populations
Following opening remarks by Dr Michael Makanga, EDCTP Executive Director, and the author's overview of the EDCTP COVID-19 portfolio, the two-day webinar kicked-off with the session on surveillance and response strategies. The ITAIL-COVID-19, TRACE and CSIGN consortia presented their work. The ensuing panel discussion included the investigators from the AFRICOVER, MozCOVID and COVADIS consortia. They shared their experiences on what is being done to ensure their findings are used by governments.
Based on well-elaborated communication plans, these projects shared findings with relevant national policy-making bodies. They also engaged communities to tackle stigma and increase acceptance of science-based response measures. It was flagged as a major issue that while some countries have the capacity to generate genomic surveillance data, there is need to strengthen capacity to share such data between countries. A possibly continent-wide database could serve as reference depository for national public health institutions.
Surveillance and response strategies


On the second day of the webinar, the third session focused on COVID-19 in the context of the larger infectious disease burden. Presentations were given on the work of the HALT-COVID-19, TREATS-COVID and ITAIL-COVID-19 consortia. For the panel discussion the speakers were joined by representatives of the RE-BCG-CoV-19, Africa SuitcaseLab and AfriDx nconsortia.
The pandemic caused a narrow focus on COVID-19 and led to disruptions in health service delivery including a decrease in care for patients with tuberculosis (TB), malaria, HIV and Neglected Infectious Diseases. For example, services were disrupted where COVID-19 cases overburdened health systems, while pandemic response measures limited the
usual programmatic activities. Some studies also indicated
that increased mortality has been driven by interruption of ART for HIV patients, late diagnosis and treatment of new TB cases, and curtailment of mosquito net distribution for malaria.
To address the major disruption of services to other patients, panelist insisted on the need to promote local production of reagents and other products required for prevention, diagnosis and treatment of major infectious diseases. Another point that was made, is the need to encourage and support research to possibly repurpose drugs; collaboration with existing disease programs may enable such research. As TB and HIV patients are at an increased risk of severe COVID-19, speakers emphasised the need for COVID-19 projects to integrate screening for other diseases.
COVID-19 and other infectious diseases
The last session featured presentations by Covid-19 HCW, ImmunoCoV and AIDCO. investigators who were joined by representatives of the BCG-COVID-RCT and Anticov consortia for a panel discussion that explored the key emerging questions and knowledge gaps specific to sub-Saharan Africa.
The panel noted the need to assess the prevalence of asymptomatic cases and study the biological mechanisms behind the observed non-progression to symptoms.
Similarly, data accuracy required further study as there is uncertainty about the actual numbers of infections or deaths e.g. in rural versus urban areas, or from country to country.
Importantly, as the currently developed vaccines were or will be approved based on data collected mainly outside Africa, the speakers pointed to the need to have comparative or complementary regional vaccine efficacy and safety studies involving sub-Saharan African populations.
Emerging questions and knowledge gaps specific to sub-Saharan Africa
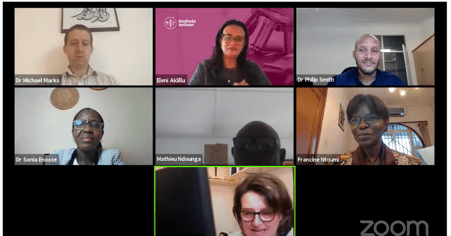
Session 1 - Panel Discussion with Michael Marks, Philip Smith, Sonia Enosse, Mathieu Ndounga and Annette Erhart, Moderated by Eleni Aklillu and Francine Ntoumi.
The presentations can be found here.
The webinar was recorded and can be found on YouTube: Day 1 and Day 2.
Download the brochure EDCTP2 portfolio: COVID-19 collaborative clinical research (PDF).
Visit the public portal of the EDCTP grants system for detailed information on the projects.
EDCTP activated this mechanism in April 2020 to support collaborative clinical research studies in sub-Saharan Africa to manage and/or prevent the spread of the COVID-19 epidemic. As of March 2021, twenty-six projects are ongoing in 22 countries across sub-Saharan Africa. One year on from the declaration of COVID-19 as a pandemic, many research questions have been answered, many others are still under investigation and new questions are emerging.
The objectives of the webinar were threefold. First, to share experience and study findings among the EDCTP COVID-19 research consortia. Secondly, to discuss new emerging questions and the current public health priorities in sub-Saharan Africa. Thirdly, to inform the global research community and stakeholders about this EDCTP-funded COVID-19 research.
The webinar was divided in four sessions focused on, respectively:
1) surveillance and response strategies;
2) special or key populations;
3) COVID-19 and other major infectious diseases; and
4) new emerging questions and knowledge gaps specific
to sub-Saharan Africa.

The second session included presentations from the periCOVID-Africa, COVAB and COREP consortia, and concluded with a panel discussion where the three presenters were joined by investigators from Profile-Cov and RADIATES consortia.
The key point made in this session was the need to address the knowledge gaps regarding risk, transmission and immunity in pregnant women and their infants. A call was made to increase the inclusion of pregnant women in ongoing and planned clinical studies but also in the planned safety studies that will accompany vaccine roll-out programmes. A lack of vaccines safety data for pregnant women may affect vaccines acceptance.
Speakers also explored the broadening definition of key populations in the context of COVID-19. Patients with non-communicable conditions such as obesity would fall under the definition but also varying categories of front-line workers. Depending on the socio-economic situation in various countries front-line workers could include police agents or bankers. The issue of stigma was mentioned again. Speakers stressed the impact that mistrust and stigma may have on research response activities, by keeping key populations away from studies.
Key populations
Following opening remarks by Dr Michael Makanga, EDCTP Executive Director, and the author's overview of the EDCTP COVID-19 portfolio, the two-day webinar kicked-off with the session on surveillance and response strategies. The ITAIL-COVID-19, TRACE and CSIGN consortia presented their work. The ensuing panel discussion included the investigators from the AFRICOVER, MozCOVID and COVADIS consortia. They shared their experiences on what is being done to ensure their findings are used by governments.
Based on well-elaborated communication plans, these projects shared findings with relevant national policy-making bodies. They also engaged communities to tackle stigma and increase acceptance of science-based response measures. It was flagged as a major issue that while some countries have the capacity to generate genomic surveillance data, there is need to strengthen capacity to share such data between countries. A possibly continent-wide database could serve as reference depository for national public health institutions.
Surveillance and response strategies

On the second day of the webinar, the third session focused on COVID-19 in the context of the larger infectious disease burden. Presentations were given on the work of the HALT-COVID-19, TREATS-COVID and ITAIL-COVID-19 consortia. For the panel discussion the speakers were joined by representatives of the RE-BCG-CoV-19, Africa SuitcaseLab and AfriDx nconsortia.
The pandemic caused a narrow focus on COVID-19 and led to disruptions in health service delivery including a decrease in care for patients with tuberculosis (TB), malaria, HIV and Neglected Infectious Diseases. For example, services were disrupted where COVID-19 cases overburdened health systems, while pandemic response measures limited the
usual programmatic activities. Some studies also indicated that increased mortality has been driven by interruption of ART for HIV patients, late diagnosis and treatment of new TB cases, and curtailment of mosquito net distribution for malaria.
To address the major disruption of services to other patients, panelist insisted on the need to promote local production of reagents and other products required for prevention, diagnosis and treatment of major infectious diseases. Another point that was made, is the need to encourage and support research to possibly repurpose drugs; collaboration with existing disease programs may enable such research. As TB and HIV patients are at an increased risk of severe COVID-19, speakers emphasised the need for COVID-19 projects to integrate screening for other diseases.
COVID-19 and other infectious diseases
The last session featured presentations by Covid-19 HCW, ImmunoCoV and AIDCO. investigators who were joined by representatives of the BCG-COVID-RCT and Anticov consortia for a panel discussion that explored the key emerging questions and knowledge gaps specific to sub-Saharan Africa.
The panel noted the need to assess the prevalence of asymptomatic cases and study the biological mechanisms behind the observed non-progression to symptoms. Similarly, data accuracy required further study as there is uncertainty about the actual numbers of infections or deaths e.g. in rural versus urban areas, or from country to country.
Importantly, as the currently developed vaccines were or will be approved based on data collected mainly outside Africa, the speakers pointed to the need to have comparative or complementary regional vaccine efficacy and safety studies involving sub-Saharan African populations.
Emerging questions and knowledge gaps specific to sub-Saharan Africa


To consolidate its work in community engagement, EDCTP has created a working group involving experts from the General Assembly and the Scientific Advisory Committee. Currently, the working group is reviewing EDCTP-funded research through the lens of community engagement. It will develop recommendations to strengthen EDCTP’s strategy in this area, taking into consideration the orientation of the future third EDCTP programme, i.e. its expected increased focus on late-stage product development, vulnerable populations and emerging and re-emerging infections.
Next steps to further community engagement
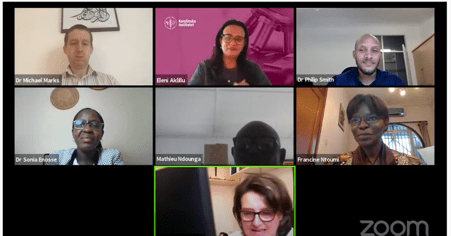
Session 1 - Panel Discussion with Michael Marks, Philip Smith, Sonia Enosse, Mathieu Ndounga and Annette Erhart, Moderated by Eleni Aklillu and Francine Ntoumi.
The presentations can be found here.
The webinar was recorded and can be found on YouTube: Day 1 and Day 2.
Download the brochure EDCTP2 portfolio: COVID-19 collaborative clinical research (PDF).
Visit the public portal of the EDCTP grants system for detailed information on the projects.