
Enhancing drug-safety monitoring


scroll down
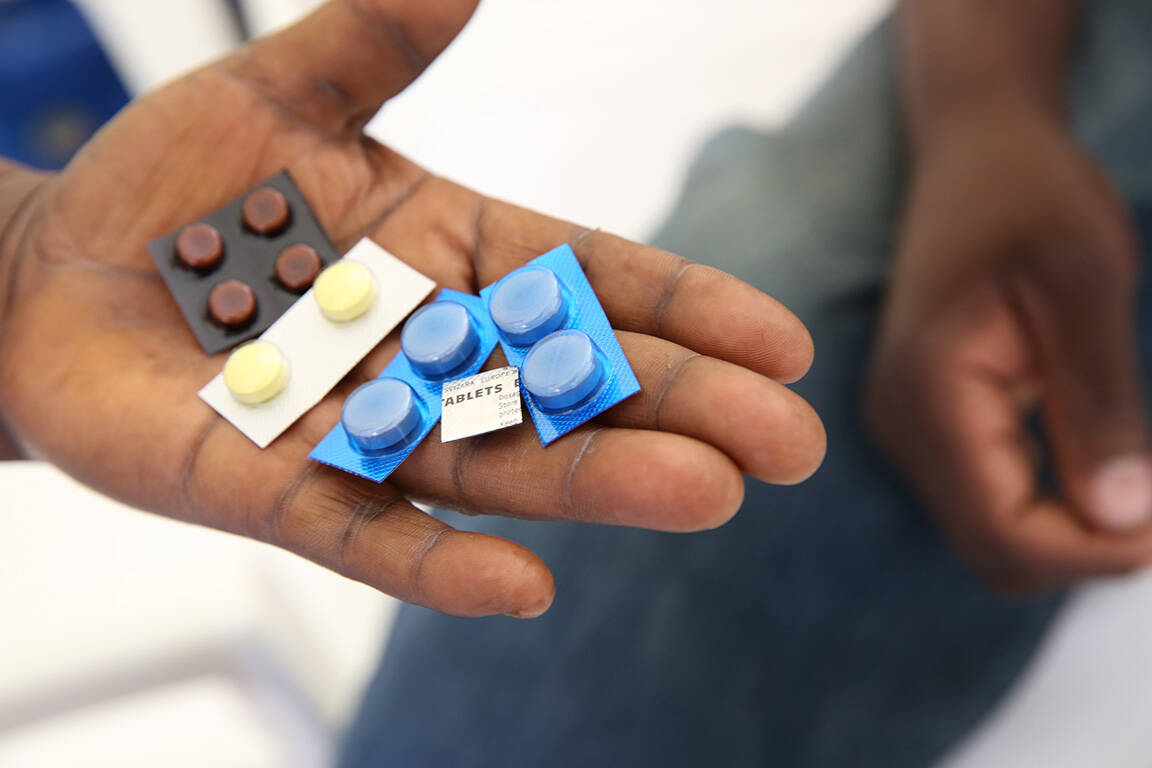
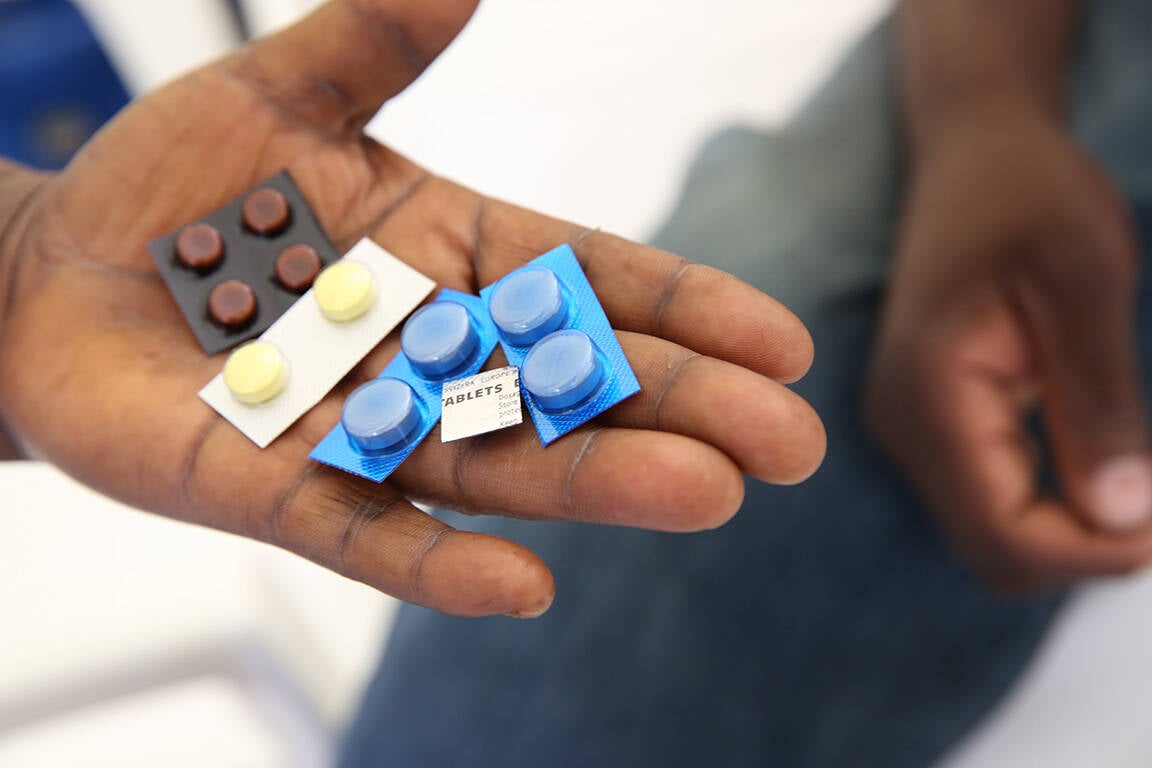
Increasing access to new medications in sub-Saharan Africa is highlighting the need for effective national drug monitoring systems. Protecting participants in clinical trials, and the general public once new medicines are introduced, requires effective systems to monitor for potential adverse reactions (pharmacovigilance). Comprehensive systems need to be in place to identify reactions when they occur, to investigate them to determine whether they are truly related to a new intervention, and to take action if public health is threatened.

Three major international projects also focus on pharmacovigilance. EXIT-TB aims to accelerate the translation of research into policy and practice through implementation of the Evidence-Based Multiple Focus Integrated Intensified TB Screening Package (EXIT-TB) in the East African region. The EXIT-TB project is implemented under the platform of East Africa Consortium for Clinical Research (EACCR), in partnership with the University College London (UCL), Liverpool School of Tropical Medicine (LSTM) and University of Bergen.
The PAVIA project is focusing on improving pharmacovigilance systems in Eswatini, Ethiopia, Nigeria and Tanzania. It strengthens links between national regulatory authorities (NRAs), national public health programmes and local medical research institutes. Focusing on multidrug-resistant TB (MDR-TB), it will build links between national TB control programmes and NRAs, and build local capabilities to detect and investigate possible adverse reactions to MDR-TB treatment.
The PROFORMA project has brought together Ethiopia, Tanzania, Kenya and Rwanda. It has also undertaken baseline national assessments together with PAVIA and is building pharmacovigilance capacity across the four countries by strengthening the clinical trial regulatory capacity for regional medicine regulatory harmonisation. It has also assessed current pharmacovigilance teaching in East Africa in order to inform curriculum development. The project is initially focusing on mass drug administration campaigns and vaccine introductions as a platform to develop pharmacovigilance capacity.
We reached out to PROFORMA’s coordinator, Professor Eleni Aklillu, to hear about the challenges and successes of building pharmacovigilance in the East African region.
The SPaRCS project is supporting coordinated development of pharmacovigilance capacity in four southern African states – Eswatini, Namibia, South Africa and Zimbabwe – helping to build a sub-regional community of practice to promote coordinated capacity development.
The EAPI project is addressing the shortage of capacity within national regulatory agencies to conduct pharmacovigilance activities. EAPI is a partnership between the Pharmacy and Poisons Board – the national regulatory agency in Kenya – and the University of Nairobi. The partners are jointly developing e-learning materials to enable academic staff to contribute to practical national pharmacovigilance activities. The virtual training tools could also be used to develop capacity across other countries in East Africa.
Many countries in sub-Saharan Africa have weak infrastructure for pharmacovigilance. Building pharmacovigilance capacity is part of several EDCTP-funded projects building national regulatory capacity in clinical research across the four regions of Africa. For example, the Africlinique project is building pharmacovigilance capacity in countries in Central Africa, starting with Cameroon and the Republic of the Congo, in collaboration with EU institutions and regional bodies such as the African Vaccine Regulatory Forum (AVAREF) and the EDCTP-funded Central African Network on Tuberculosis, HIV/AIDS and Malaria (CANTAM).
scroll down
Enhancing drug-safety monitoring
Nuraan Fakier, Michelle Singh and Thomas Nyirenda

EDCTP funding of ethics and regulatory activities
Since 2005 EDCTP has dedicated its efforts to ensure that all sub-Saharan African countries hosting clinical trials have functional and effective ethics and regulatory review structures at institutional, national and regional levels. EDCTP continues to develop its funding strategy to build upon successes and lessons learned, and through working with partners such as Africa CDC, WHO-AFRO and AUDA-NEPAD to achieve this.
TESA
The Trials of Excellence in Southern Africa (TESA) was set up in 2009 and is investing in the establishment of an accredited referral data management centre. It already has three referral laboratories in different countries within Southern Africa which serve as training platforms. Experienced TESA research centres also function as training platforms for clinical research studies using an on-the-job training approach.
Currently, most successful research programs in Africa are being carried out by international consortia or networks. Therefore, TESA collaborates with various other networks that operate in the region, e.g. the MOSASWA (Mozambique, South Africa, Swaziland Cross-Border Malaria Initiative), ARISE (Africa Research Initiative and Support Network) and ECRIN (European Research Infrastructure). In addition, TESA has been very active and successful in bringing Namibia, Angola and Eswatini within the network.
EACCR
The East African Consortium for Clinical Research (EACCR) was set up in 2009. It has been involved in projects on the retention of HIV-infected mothers and their babies in HIV care, and in HIV pharmacovigilance in young people. On malaria, it has been involved in epidemiological studies and clinical trials of artemisinin-based combination therapy and antimalarial resistance. Additionally, the network is successfully collaborating with the East African Community Health Research Commission (EACHRC) on regional sharing of research evidence and actively facilitating researcher-policymaker dialogue.
The network also invests in preparing resource-limited clinical research sites to conduct clinical trials on the infectious diseases that burden the region. EACCR has developed an e-learning centre that hosts peer-reviewed and globally applicable short courses required for high-quality clinical studies. This is currently being strengthened by the addition of a regionally harmonised training course to qualify as Clinical Research Associate.
Importantly, it established a regional pool of trained clinical trial monitors to support a high quality of clinical trials across the region. EACCR’s reciprocal monitoring scheme is an innovative, practical and affordable scheme for quality management of health research. The monitors are qualified to be independent monitors, some also having accreditation with the Association of Clinical Research Professionals. To date, several clinical research studies (vaccine trials, drug safety studies, epidemiological studies and longitudinal intervention studies) across Africa have been monitored by EACCR monitors.
Pandemic ethics
Strengthening ethics and regulatory systems is not only essential for conducting trials where the burden of disease is highest, but is also critical to conducting research during epidemics, such as the COVID-19 pandemic. The pandemic has highlighted the importance of rigorous and efficient ethics and regulatory approval. Ethics committees, supported by EDCTP, have drafted Standard Operating Procedures for expedited review and prepared an Outbreak Preparation Plan, which have, facilitated the procedures for roll-out of COVID-19 vaccines.
Moreover, the network keeps investing in training activities to strengthen pharmacovigilance, national regulatory authorities and ethics committees, a regional capacity gap which is being addressed with urgency.
Addressing the gender gap in clinical research capacity is another priority area for CANTAM which led it to develop a strategy to involve more women in medical research. The network initiated a project entitled ‘Women and Science’ which promotes ‘Girls in science’ in local schools and aims to mitigate the marginalisation of female scientists through the establishment of a career development fellowship for post-doctoral female researchers in Central Africa. This project is being rolled out across Central Africa via the EDCTP-funded fellowship programme WISE which aims to strengthening gender capacity in clinical research within the CANTAM network.
Introducing ethics review legislation
Whilst capacity has improved greatly, there remain some African countries where legislation for clinical trials does not exist. The “Biomedical Ethics and Regulatory Capacity Building Partnership for Portuguese Speaking African Countries” (BERC-Luso project) (https://www.berc-luso.com) aims to establish and develop well-grounded, sound, robust and sustainable ethics and regulatory capacities in five Portuguese-speaking African partner countries (Angola, Cape Verde, Guinea-Bissau, Mozambique, Sao Tome and Principe). The project is drafting national legislation governing the conduct of clinical trials in order to provide the conditions for the development of biomedical research and specifically clinical trials in these countries as well as the adoption of international best practices, thus assuring populations’ protection and countries’ development.
EDCTP1 funded the Mapping African Research Ethics and Drug Regulatory Capacity (MARC) project. The MARC project (https://www.healthresearchweb.org/) was a three-year initiative, which aimed to develop an interactive resource map of Africa’s HRECs and provide a web-based platform to increase contact and communication between African HRECs. A secondary objective was to map Medicines Regulatory Authorities (MRAs) and facilitate better links between MRAs and HRECs. To that end, MARC and the Health Research Web teams developed an information management system named the Research for Health and Innovation Organiser (RHInnO Ethics) (https://www.ethixpert.org.za/rhinno-ethics/). RHInnO Ethics was first developed by the Council on Health Research for Development (COHRED) and is now owned, managed and coordinated by EthiXPERT.
Ethics committees are increasingly adopting online review methods and programmes, such as RHInnO Ethics. RHInnO Ethics produces important performance indicators for ethics committees, sends real-time alerts and reminders to researchers, reviewers and administrators hence increases efficiency. It can be used to report on and monitor Serious Adverse Events (SAEs) thus enhancing the ethics committee’s pharmacovigilance practices. It also has a ‘national’ version, that enables national ethics committees to provide accreditation and to monitor the quality and performance of institutional committees. The overall objective of the RHInnO Ethics platform is to provide national ethics committees and institutional review boards with a tool to collect, collate, analyse and display information on health and other research conducted in their countries. Of course, implementing innovative systems in sub-Saharan Africa is not without challenges, which include internet connectivity, cost and maintenance of systems, equipment and IT support.
Through its funding, EDCTP has supported national ethics committees to implement online systems such as RHInnO Ethics. The investment in online solutions has helped to streamline ethics review (hard copies of protocols are no longer required) and has made remote review easier, especially with committees operating virtually during the COVID-19 pandemic.
Regional and (inter)national collaboration: Pan-African Clinical Trials Alliance (PACTA)
Many institutions in sub-Saharan Africa lack research laboratories that are ISO accredited and comply with standards of Good Clinical and Laboratory Practice. The establishment of the NoEs provided an opportunity for systematic infrastructural upgrades in order to achieve compliance with international requirements
In 2012, EDCTP initiated a laboratory strengthening project within the four regional NoEs to systematically develop 24 clinical research and public health laboratories towards internationally recognised accreditation. The WHO-AFRO Stepwise Laboratory Improvement Process Towards Accreditation (SLIPTA) was used to benchmark and develop the selected laboratories towards accreditation. To date, seven laboratories in the networks have successfully achieved and/or maintained their ISO 15189:2012 accreditation. These are: CHU A. Le Dantec in Senegal, Medical Research Council The Gambia in The Gambia; Kenya Medical Research Institute/Centre for Disease Control (Kisian Campus) in Kenya; Medical Research Council–Uganda Virus Research Institute in Uganda; the Central Tuberculosis Reference Laboratory in Tanzania; the Manhiça Health Research Centre in Mozambique; and the Nigerian Institute For Medical Research. These accredited laboratories linked to the networks of excellence are now able to compete globally for high-quality research projects adhering to international standards.
Increasing access to new medications in sub-Saharan Africa is highlighting the need for effective national drug monitoring systems. Protecting participants in clinical trials, and the general public once new medicines are introduced, requires effective systems to monitor for potential adverse reactions (pharmacovigilance). Comprehensive systems need to be in place to identify reactions when they occur, to investigate them to determine whether they are truly related to a new intervention, and to take action if public health is threatened.

Many countries in sub-Saharan Africa have weak infrastructure for pharmacovigilance. Building pharmacovigilance capacity is part of several EDCTP-funded projects building national regulatory capacity in clinical research across the four regions of Africa. For example, the Africlinique project is building pharmacovigilance capacity in countries in Central Africa, starting with Cameroon and the Republic of the Congo, in collaboration with EU institutions and regional bodies such as the African Vaccine Regulatory Forum (AVAREF) and the EDCTP-funded Central African Network on Tuberculosis, HIV/AIDS and Malaria (CANTAM).