EDCTP Prizes recognise the achievements of outstanding researchers and research teams. During each EDCTP Forum, four international prizes are awarded to promote scientific research and Africa-European collaboration.


The AETBC team
The 2020 EDCTP Prize winners were announced at the Tenth EDCTP Forum:
Dr Pascoal Mocumbi Prize winner: Professor Sir Alimuddin Zumla
Sir Alimuddin (Ali) Zumla is ranked as the world’s top communicable diseases expert and has authored 22 medical textbooks and contributed to 800 publications worldwide. Sir Ali Zumla is renowned for his empowerment of African and European scientists.
“You cannot say that because you are a woman, and you have these other roles to play, you are not going to strive for excellence. Hard work with excellence always pays off.”
— Prof. Margaret Gyapong


scroll down
About the EDCTP Prizes
The biennial EDCTP Prizes recognise outstanding individuals and research teams from Africa and Europe who have made significant achievements in their research field. Additionally, the awardees will have made major contributions to strengthening clinical research capacity in Africa and supporting South-South and North-South networking.
The Prizes are part of the EDCTP programme which is supported by the European Union under Horizon 2020, it’s Framework Programme for Research & Innovation.
“I think the success of AE-TBC shows that African institutions can lead international research effectively, that long-term relationship between individuals in different institutions across the globe, and in this case across Africa, is very important.”
— Prof. Gerhard Walzl, AETBC coordinator




Outstanding Research Team Prize winner: African-European Tuberculosis Consortium (AETBC)
The AETBC consortium is an international multisite group of African and European researchers to investigate the use of host biosignatures for the diagnosis of active TB disease.
Outstanding Female Scientist Prize winner: Professor Margaret Gyapong
Professor Margaret Gyapong is interested in research impact and has been a leader in this area bringing together the experiences of research institutions in Africa, Asia and Europe. In 25 years, Prof. Gyapong has risen through the ranks of the research ladder to become a seasoned and internationally renowned scientist.
“I feel extremely humbled and honoured and to receive this very prestigious prize – especially since Dr Pascoal Mocumbi is globally distinguished and respected for his exemplar role model scientific and political work.”
— Prof. Sir Alumuddin Zumla
Prof. Graeme Meintjes
Scientific Leadership Prize winner: Professor Graeme Meintjes
“The award of this prize from EDCTP is a highlight of my career to date. It is a recognition of the work that our research group has done.”
— Prof. Graeme Meintjes






Prof. Margaret Gyapong

Prof. Sir Alimuddin Zumla

EDCTP Prizes recognise the achievements of outstanding researchers and research teams. During each EDCTP Forum, four international prizes are awarded to promote scientific research and Africa-European collaboration.
scroll down
Michelle Helinski, Ana Lúcia Weinberg, Ian Jones


EDCTP - Public consultation: Global Roadmap for research and development for TB vaccines (13 January 2021)
AIGHD - EDCTP asks AIGHD to develop road map for tuberculosis vaccines (28 August 2020)
EDCTP - Developing a Global TB Vaccine R&D Roadmap
(02 March 2020)
About the EDCTP Prizes
The biennial EDCTP Prizes recognise outstanding individuals and research teams from Africa and Europe who have made significant achievements in their research field. Additionally, the awardees will have made major contributions to strengthening clinical research capacity in Africa and supporting South-South and North-South networking.
The Prizes are part of the EDCTP programme which is supported by the European Union under Horizon 2020, it’s Framework Programme for Research & Innovation.
The road to End TB
If all stakeholders collectively and collaboratively focus on the priorities identified in this Roadmap, progress will be accelerated. The unprecedented speed at which COVID-19 vaccines have been developed holds important lessons that can be applied to TB vaccines.
One hundred years after the BCG vaccine, there is real hope that disadvantaged populations around the world can finally gain the benefits of TB prevention and cure through the use of vaccination.
New TB vaccines will fulfil multiple roles in TB control but a stronger and more diverse TB vaccine pipeline needs to be build. It is equally important to begin preparing the ground for the introduction of new TB vaccines. An end-to-end perspective needs to be adopted. Clinical development must be informed by a sound understanding of national decision-making and implementation constraints, while licensing must be swiftly followed by efficient programmatic implementation.
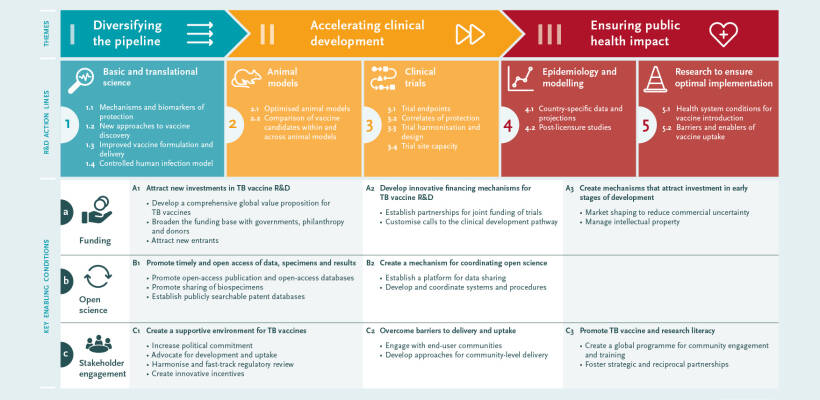
Enabling factors
Global efforts are required to encourage ‘open science’ with greater sharing of data and specimens, facilitated by the establishment of biobanks and data platforms. A failure to publish data, particularly negative results, can lead to unnecessary duplication of efforts and research being carried out on approaches unlikely to be successful.
A wide range of stakeholders needs to be engaged, including global agencies, industry, regulatory authorities, national decision-makers and local communities. Additional investment in TB vaccine R&D, awareness of the need for and possibilities of new TB vaccines, and community support for vaccination are the main goals of this engagement.
Three cross-cutting enabling factors were also identified: 1) funding; 2) open science, and 3) stakeholder engagement.
TB research is underfunded globally and TB vaccine research is particularly poorly resourced. Interest in funding TB vaccine R&D needs to be increased and more funders need to be involved. Greater coordination across funders could help to increase R&D productivity and accelerate clinical evaluation. To create greater commercial interest in TB vaccine R&D, innovative funding (“push”) mechanisms need to be combined with market-shaping (“pull”) approaches, such as advance market commitments.


Priorities
The Roadmap consultation identified three priority areas:1) diversifying the pipeline, 2) accelerating clinical development, and 3) ensuring public health impact.
The TB vaccine pipeline is not well-stocked and lacks diversity. Early-stage R&D has focused on a limited number of Mycobacterium tuberculosis (Mtb) antigens and on a very specific type of immune response. There is a need to:
- Increase the diversity of the pipeline by focusing on a wider range of antigens, immune responses and delivery mechanisms.
- Improve understanding of correlates of protection and identify biosignatures that correlate with vaccine-induced protection.
- Improve understanding of mucosal immune responses in the lung and how they relate to systemic responses.
- Explore the potential use of controlled human infection models.
Clinical evaluation of TB vaccines is slow, costly and high-risk. In part, this reflects the fact that efficacy in animal models is not a good predictor of efficacy in people, making it difficult to prioritise candidates for clinical evaluation. A further important barrier is the lack of well-defined correlates of protection or proxy measures of efficacy. Demonstration of efficacy therefore requires large trials with prevention of disease as the primary endpoint. There is a need to:
- Optimise animal models by “back-translating” findings from successful clinical trials.
- Optimise alternative endpoints, by establishing the relationship between prevention of disease and other possible endpoints (particularly prevention of infection and prevention of recurrence).
- Harmonising and standardising trial protocols, and exploring innovative trial designs to improve efficiency.
- Last but not least, clinical trial capacity must be built in high-burden countries.
A key milestone in new product development is licensing approval, but this does not by itself deliver public health benefit. Multiple barriers may hamper the timely implementation of a newly licensed TB vaccine. Key challenges include limited understanding of how a new vaccine would be used within different countries, as well as the lack of investment cases to support national and global decision-making on procurement and implementation. To address these issues there is a need to:
- Collect data to model potential impacts of vaccine use and develop country-use scenarios and investment cases
- Undertake activities to prepare countries for evaluation of newly licensed vaccines and rapid implementation once recommended locally.
- Identify and develop strategies to address potential barriers to implementation, including community resistance to TB vaccination.
- Post-licensure studies should be planned to establish vaccine effectiveness.
Dr Michael Makanga, EDCTP Executive Director: “The main drive for investing in this very consultative new global TB vaccine roadmap is our enduring commitment to a concerted global R&D effort that will ultimately end TB. This timely Roadmap may leverage recent advances and new opportunities arising from lessons learnt from other diseases such as COVID-19”.
The 2020 EDCTP Prize winners were announced at the Tenth EDCTP Forum:
