Johanna Roth
Filling the gap in funding for research on cryptococcal meningitis with advanced HIV disease
Dr Angela Loyse
Coordinator of the DREAMM project


Mortality associated with HIV infection is much higher in Africa than in high-income countries. Cryptococcal meningitis is an important cause of morbidity and mortality among people with advanced HIV disease and accounts for an estimated 15% of all AIDS-related deaths globally, of which three quarters occur in sub-Saharan Africa. In 2014, it is estimated that 223,100 cases of cryptococcal meningitis resulted in 181,000 deaths.
Despite its major burden in sub-Saharan Africa, cryptococcal meningitis remains under-studied and under-funded. The 2019 G-FINDER annual report highlighted the fact that the bulk of non-USA funding was routed through EDCTP, and accounted for 85% of global funding on clinical development.
scroll down
AMBITION-cm: Improving treatment of HIV-associated meningitis
The AMBITION-cm team has been evaluating an alternative formulation of amphotericin B, delivered in tiny lipid-based packages (liposomes), which would be more suitable for resource-poor settings. A phase II study showed that a single high dose of liposomal amphotericin B (AmBisome®) (liposomal amphotericin B) given with 14 days of flucytosine and fluconazole is non-inferior to the current standard of care of seven days of amphotericin B plus seven days of flucytosine, followed by seven days of fluconazole, in the treatment of HIV-associated cryptococcal meningitis (CM).
The AMBITION-cm trial is the largest HIV-associated cryptococcal meningitis treatment trial ever undertaken. The results of this trial, which were presented at the International AIDS Society (IAS) conference in July 2021, confirm that a simple amphotericin B-based therapy could transform treatment of one of the most important causes of HIV-associated mortality in Africa.
DREAMM: Improving management of meningitis in HIV-infected adults
There is growing evidence, from EDCTP-funded and other trials, that early screening for cryptococcal infections and prompt treatment can save lives. However, take-up of diagnostics has so far been limited, in part because of the practical difficulties of implementing new procedures in existing healthcare systems.
The DREAMM project aimed to embed innovative meningitis diagnostic practices into routine health care. One key aim of the project was to generate data on an upgraded version of a lateral flow diagnostic for cryptococcal infections, the Cryptococcal Antigen Lateral Flow Assay (CrAG LFA) test, a diagnostic test that is heat-stable, inexpensive, requires minimal training and has been shown to be cost-effective in resource-limited settings. The test provides an indication of the severity of infection and the need for more intensive care.
In collaboration with healthcare staff, the DREAMM team developed new clinical pathways and decision-making procedures. Once these new procedures were finalised, staff was trained in using them. The project then evaluated whether the DREAMM diagnostic and treatment algorithm reduced the delays in assessment and treatment, and led to improved survival.
DREAMM has been designed to generate both evidence and resources directly relevant to health policymakers. It was shown that the DREAMM model of care appears to be effective, safe and generalisable. Two-week mortality data after the implementation of the DREAMM algorithm was 26%, compared to 48% in the observation phase (before algorithm implementation). This is comparable to clinical trial outcomes, despite a high proportion with abnormal mental status at baseline (which is a poor prognostic marker). The mortality reduction at 10 weeks was less apparent (42% in the implementation phase compared to 55% in the observation phase) and work needs to be done to optimise post-hospital care. The study also produced training and other materials to support implementation elsewhere, enabling the new procedures and diagnostics tools to be readily introduced in other settings.


Coordinator of the TRIP project
Dr Godfrey Sayoki Mfinanga
TRIP: Translating research into practice
This study built on the earlier EDCTP-funded REMSTART project: Reduction of early mortality among HIV-infected subjects starting antiretroviral therapy: a randomised trial. The project evaluated a health service strategy designed to reduce the high early mortality associated with antiretroviral therapy in Africa.The TRIP intervention package involved 1) Cryptococcal meningitis screening using the CrAg test plus pre-emptive treatment with fluconazole and 2) weekly mobile telephone messaging as a home support for one month followed by monthly telephone consultations for the next 3 months. TRIP aimed to determine the feasibility, cost-effectiveness, and impact of scaling up the REMSTART intervention in 24 urban and 12 rural settings in Tanzania. Results showed that, the trend in probability of death over 12 months was about 2 times higher among CrAg positive patients than in CrAg positive asymptomatic participants, and about 3 times higher among CrAg positive patients than in CrAg negative patients.
5FC HIV-Crypto: Improved flucytosine formulation for the treatment of meningitis in advanced HIV disease
This project investigates a simpler, sustained-release formulation of flucytosine for the treatment of cryptococcal meningitis and its evaluation in a phase 1/2 trial, to make treatment affordable and accessible to more people. The phase 1 study will be undertaken in South Africa, followed by the phase 2 studies in Tanzania and Malawi. The project also aims to strengthen local capacities in conducting clinical trials and focused work will be carried out to facilitate the scale-up of flucytosine implementation according to WHO guidelines.
The current recommended induction treatment consists of a one-week course of amphotericin B deoxycholate and flucytosine, followed by 1 week of fluconazole. This regimen is however not widely available in most African countries as access to both amphotericin B and flucytosine is limited. Even when registered, amphotericin B is rarely used in African settings because it has to be given intravenously over one week and can trigger severe toxic reactions so patients require specialist monitoring.
In addition to improving treatment regimens and access to essential drugs, much needs to be done to improve timely diagnosis of cryptococcal infections and implementing the right procedures in healthcare systems. This is exactly what four EDCTP-funded projects on cryptococcal meningitis are working on.

Coordinator of the AMBITION-cm study
Professor Joe Jarvis
Read more:
Improving treatment of HIV-associated meningitis
Professor Joe Jarvis
Coordinator of the AMBITION-cm study

scroll down
Another version of amphotericin B, liposomal amphotericin B, is widely used in high-income settings, and we showed that this formulation is less toxic and could be very suitable for use in low-resource settings. However, it is very expensive. That’s why we wanted to study whether a single high-dose of liposomal amphotericin B would work as well as the 7-day treatment regimen. This was initially studied in a phase 2 trial, followed by the phase 3 AMBITION-cm study.
The results of the AMBITION-cm study are very encouraging. The single high-dose arm of the study performed as well as the 7-day arm, and was less toxic. In settings where intravenous treatment not seldom leads to complications, this would make treatment much safer and easier. We designed the trial specifically for low and middle income countries, and African countries in particular, as this is where the burden of disease is highest.
Although science had progressed relatively fast on this topic, access to treatment remains a major concern. Availability is an issue for both Amphotericin B and flucytosine, registration on a national level is often not in place, and procurement is difficult. I hope these issues can be solved through collaborations throughout the chain, and our research team is working with all stakeholders to improve access to treatment for those who need it most.“
AMBITION-cm

Project: AMBITION-cm trial
Project lead: Professor Joe Jarvis, London School of Hygiene and Tropical Medicine, UK
Countries involved: Botswana, France, Malawi, South Africa, Uganda, United Kingdom, Zimbabwe
Target population(s): Adults with HIV
Year funded: 2017
End date: 31 October 2021
EDCTP funding: €10 M
Read more:
Improving management of meningitis in HIV-infected adults
Dr Angela Loyse
Coordinator of the DREAMM study

scroll down
“Ever since I started doing research on infections that present themselves with HIV, I have felt a growing need to look at the actual implementation of best practices in routine care. HIV-related infections affecting the central nervous system, including cryptococcal meningitis, are often undiagnosed and untreated causing 100,000s potentially preventable deaths each year. That’s why with our DREAMM project, we wanted to develop sustainable and effective implementation interventions and strategies with available tests and treatments that will have a lasting impact on local health systems.
The DREAMM project included three steps: observation, training and implementation. First, rapid appraisals were introduced as a means to understand the existing health system for central nervous system infections in hospitals in Malawi, Tanzania and Cameroon. We organised focus group sessions with all involved, from doctors and nurses to laboratory technicians and porters. Then we mapped out what happens when a sick person living with HIV comes in: What is the routine pathway? One of the things we found for example was that many of the patients did not get a lumbar puncture and CD4 counts and viral loads were often not performed. The patients were not diagnosed, and instead received empirical treatment. In the DREAMM site in Tanzania, the system was subsequently changed such that patients would be guided into a dedicated ‘meningitis room’ and received rapid diagnostic testing as soon as possible, as speed is essential in treating HIV-related central nervous system infection.
Second, we developed a free online educational programme for frontline healthcare workers and laboratory technicians which has already been viewed over 4000 times. In the final project phase, the main DREAMM intervention was the implementation of an algorithm, or decision making aide, for the management of HIV-related central nervous system. This includes bedside rapid diagnostic testing (for example, all participants were tested for cryptococcal antigen (CrAg), as well as the results of the WHO-recommended regimens for cryptococcal meningitis. Alongside, the existing workforce and local leadership were empowered to optimise their own health system and deliver interventions that reduce mortality, which is key to sustainable scale-up.
Local leadership was essential in bringing hospitals to a level where they can deliver quality care for meningitis despite limited resources and ongoing challenges. Hospitals was strengthened through the DREAMM education programme, weekly communities of practice, and optimisation of clinical and laboratory pathways.
The preliminary results show that making the DREAMM implementation intervention part of routine practice and employing DREAMM implementation strategies (empowerment of local leadership and delivery of quality improvements in hospital care) could reduce mortality at 2 weeks by about 50%. Ten-week outcomes still need to be improved, so in our follow-up studies we aim to look more closely at what happens after discharge from the clinic, including linkage to care and CD4 and viral load testing, and pre-hospital care.”
DREAMM
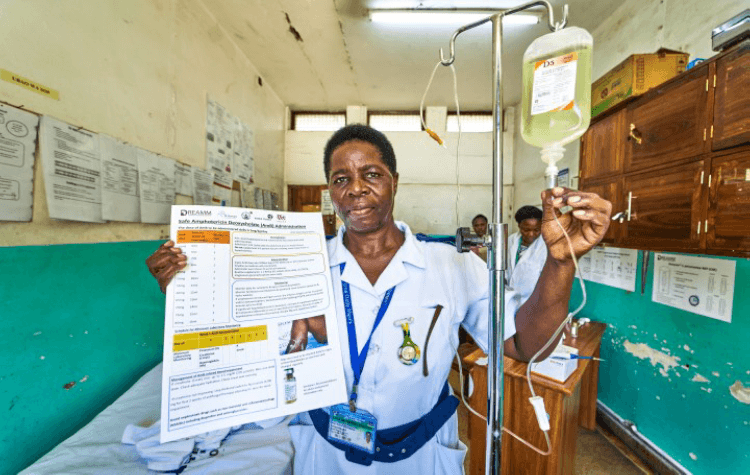
Project: DREAMM
Project lead: Dr Angela Loyse, St George’s University of London, United Kingdom
Countries involved: Cameroon, France, Malawi, Tanzania, the United Kingdom
Target population(s): Adults with HIV
Year funded: 2016
End date: 31 December 2021
EDCTP funding: €1.9 M
Read more:
Extending screening for fungal infections
Dr Godfrey Sayoki Mfinanga
Coordinator of the TRIP project

scroll down
“The TRIP-project built on the results of the EDCTP-funded REMSTART trial, which showed that a relatively low-cost intervention, based on screening for cryptococcus infections and providing home support to encourage adherence to treatment, reduced mortality by 28%. With the TRIP-project we took the cryptococcal screening into a routine setting. The DREAMM project had similar goals to implement an intervention by integrating it in standard routine care.
Both these studies need scale up. They need support from international organisations and governments to ensure availability of diagnostic tests and drugs such as flucytosine, fluconazole and liposomal amphotericin B need to be available. Thanks to UNITAID support building on our activities in the EDCTP project, for example, we were able to scale up cryptococcal meningitis screening to more than 200 facilities in Tanzania. These facilities are now routinely screening for cryptococcal meningitis and provide pre-emptive fluconazole. Critical interventions that have shown they can reduce mortality with HIV disease need support from international organisations and governments that put efforts together. This is really urgently needed to make scale-up successful and save lives.”

Project: TRIP project
Project lead: National Institute for Medical Research (NIMR), Dar es salaam, Tanzania with partners from Tanzania and the United Kingdom
Year funded: 2016
End date: 29 February 2020
EDCTP funding: €0.5 M
The presentations can be found here.
The webinar was recorded and can be found on YouTube: Day 1 and Day 2.
Download the brochure EDCTP2 portfolio: COVID-19 collaborative clinical research (PDF).
Visit the public portal of the EDCTP grants system for detailed information on the projects.

Mortality associated with HIV infection is much higher in Africa than in high-income countries. Cryptococcal meningitis is an important cause of morbidity and mortality among people with advanced HIV disease and accounts for an estimated 15% of all AIDS-related deaths globally, of which three quarters occur in sub-Saharan Africa. In 2014, it is estimated that 223,100 cases of cryptococcal meningitis resulted in 181,000 deaths.
Despite its major burden in sub-Saharan Africa, cryptococcal meningitis remains under-studied and under-funded. The 2019 G-FINDER annual report highlighted the fact that the bulk of non-USA funding was routed through EDCTP, and accounted for 85% of global funding on clinical development.
The objectives of the webinar were threefold. First, to share experience and study findings among the EDCTP COVID-19 research consortia. Secondly, to discuss new emerging questions and the current public health priorities in sub-Saharan Africa. Thirdly, to inform the global research community and stakeholders about this EDCTP-funded COVID-19 research.
The webinar was divided in four sessions focused on, respectively:
1) surveillance and response strategies;
2) special or key populations;
3) COVID-19 and other major infectious diseases; and
4) new emerging questions and knowledge gaps specific
to sub-Saharan Africa.

Key populations
“When I first started doing research on cryptococcal meningitis, the outcomes for patients with advanced HIV were extremely poor. The drug available in most LMIC countries was fluconazole, which could be given in higher doses, but this did not improve treatment outcomes. Then trials started showing that administering another drug, amphotericin B, intravenously improved patient outcomes significantly, although the requirement for intensive toxicity monitoring complicated implementation in resource-limited settings. In 2018, the ACTA-trial (Antifungal Combinations for Treatment of Cryptococcal Meningitis in Africa) proved that shortening administration of amphotericin B from 14 to 7 days and combining it with flucytosine was equally effective and less toxic. This treatment regimen then became the WHO recommended treatment regimen.
Another version of amphotericin B, liposomal amphotericin B, is widely used in high-income settings, and we showed that this formulation is less toxic and could be very suitable for use in low-resource settings. However, it is very expensive. That’s why we wanted to study whether a single high-dose of liposomal amphotericin B would work as well as the 7-day treatment regimen. This was initially studied in a phase 2 trial, followed by the phase 3 AMBITION-cm study.
The results of the AMBITION-cm study are very encouraging. The single high-dose arm of the study performed as well as the 7-day arm, and was less toxic. In settings where intravenous treatment not seldom leads to complications, this would make treatment much safer and easier. We designed the trial specifically for low and middle income countries, and African countries in particular, as this is where the burden of disease is highest.
Although science had progressed relatively fast on this topic, access to treatment remains a major concern. Availability is an issue for both Amphotericin B and flucytosine, registration on a national level is often not in place, and procurement is difficult. I hope these issues can be solved through collaborations throughout the chain, and our research team is working with all stakeholders to improve access to treatment for those who need it most.“
AMBITION-cm: Improving treatment of HIV-associated meningitis
The AMBITION-cm team has been evaluating an alternative formulation of amphotericin B, delivered in tiny lipid-based packages (liposomes), which would be more suitable for resource-poor settings. A phase II study showed that a single high dose of liposomal amphotericin B (AmBisome®) (liposomal amphotericin B) given with 14 days of flucytosine and fluconazole is non-inferior to the current standard of care of seven days of amphotericin B plus seven days of flucytosine, followed by seven days of fluconazole, in the treatment of HIV-associated cryptococcal meningitis (CM).
The AMBITION-cm trial is the largest HIV-associated cryptococcal meningitis treatment trial ever undertaken. The results of this trial, which were presented at the International AIDS Society (IAS) conference in July 2021, confirm that a simple amphotericin B-based therapy could transform treatment of one of the most important causes of HIV-associated mortality in Africa.
Session 1 - Panel Discussion with Michael Marks, Philip Smith, Sonia Enosse, Mathieu Ndounga and Annette Erhart, Moderated by Eleni Aklillu and Francine Ntoumi.
TRIP: Translating research into practice
On the second day of the webinar, the third session focused on COVID-19 in the context of the larger infectious disease burden. Presentations were given on the work of the HALT-COVID-19, TREATS-COVID and ITAIL-COVID-19 consortia. For the panel discussion the speakers were joined by representatives of the RE-BCG-CoV-19, Africa SuitcaseLab and AfriDx nconsortia.
The pandemic caused a narrow focus on COVID-19 and led to disruptions in health service delivery including a decrease in care for patients with tuberculosis (TB), malaria, HIV and Neglected Infectious Diseases. For example, services were disrupted where COVID-19 cases overburdened health systems, while pandemic response measures limited the
usual programmatic activities. Some studies also indicated that increased mortality has been driven by interruption of ART for HIV patients, late diagnosis and treatment of new TB cases, and curtailment of mosquito net distribution for malaria.
To address the major disruption of services to other patients, panelist insisted on the need to promote local production of reagents and other products required for prevention, diagnosis and treatment of major infectious diseases. Another point that was made, is the need to encourage and support research to possibly repurpose drugs; collaboration with existing disease programs may enable such research. As TB and HIV patients are at an increased risk of severe COVID-19, speakers emphasised the need for COVID-19 projects to integrate screening for other diseases.
Emerging questions and knowledge gaps specific to sub-Saharan Africa
The last session featured presentations by Covid-19 HCW, ImmunoCoV and AIDCO. investigators who were joined by representatives of the BCG-COVID-RCT and Anticov consortia for a panel discussion that explored the key emerging questions and knowledge gaps specific to sub-Saharan Africa.
The panel noted the need to assess the prevalence of asymptomatic cases and study the biological mechanisms behind the observed non-progression to symptoms. Similarly, data accuracy required further study as there is uncertainty about the actual numbers of infections or deaths e.g. in rural versus urban areas, or from country to country.
Importantly, as the currently developed vaccines were or will be approved based on data collected mainly outside Africa, the speakers pointed to the need to have comparative or complementary regional vaccine efficacy and safety studies involving sub-Saharan African populations.


Next steps to further community engagement
To consolidate its work in community engagement, EDCTP has created a working group involving experts from the General Assembly and the Scientific Advisory Committee. Currently, the working group is reviewing EDCTP-funded research through the lens of community engagement. It will develop recommendations to strengthen EDCTP’s strategy in this area, taking into consideration the orientation of the future third EDCTP programme, i.e. its expected increased focus on late-stage product development, vulnerable populations and emerging and re-emerging infections.