EDCTP2: International partnerships against
infectious disease

Dear EDCTP stakeholders,
The seventh year of implementation of the second programme of the European & Developing Countries Clinical Trials Partnership (EDCTP2) presents an opportune time to share with you the progress and achievements so far. This programme is advancing the development of diagnostics, drugs and vaccines against the most important infectious diseases affecting sub-Saharan Africa, particularly benefiting vulnerable groups such as infants, children, adolescents and pregnant women.
Dr Michael Makanga, Executive Director

The challenge
Infectious diseases remain a major cause of death and disability in sub-Saharan Africa. Despite much progress, diseases such as HIV/AIDS, malaria and tuberculosis (TB) still kill millions every year. As well as their effects on individuals, infectious diseases have a huge economic impact, in treatment costs and lost productivity.
Controlling infectious disease will therefore be core to achieving Sustainable Development Goal 3 – ensuring healthy lives and promoting wellbeing for all at all ages. Furthermore, ensuring healthy populations through effective infectious disease control will be central to achieving many other SDGs.
Control of infectious diseases requires diagnostics to detect them, drugs to treat them and vaccines to prevent them. As pathogens inevitably develop resistance to drugs, the world relies on an ongoing supply of new treatments. However, new product development is costly and risky – failure rates are high – and it is often not commercially viable for companies to develop new products for infections mainly affecting low-income countries. Innovative partnerships are therefore required to advance new product development.
To determine their safety and efficacy, new products undergo extensive evaluation, in phase I, II and III trials of increasing size. However, the results of trials in high-income countries are only a partial guide to how well products perform in other settings. Genetic differences and past history of infections, for example, can influence how people respond to drugs and vaccines. Ideally, therefore, studies need to be conducted in sub-Saharan Africa to generate locally relevant evidence.
In addition, trials undertaken to secure regulatory approval often exclude certain populations, such as children, pregnant women or people with other infections. Further studies are needed to ensure that new products are safe, effective and can be delivered in suitable formulations to these special groups.
EDCTP2 at a glance…
Duration: 2014–2024
Funding to date: €752.74 M*
Membership and reach: 16 sub-Saharan African countries are a member of EDCTP Association; 41 African countries and 244 African institutions are participating in EDCTP projects
Studies: Funded 274 clinical studies, including 124 clinical trials
Capacity-building: We are supporting 196 African researchers through fellowships; 46 ethics and regulatory projects involving 37 African countries
*As of December 2020.
EDCTP2 targets
EDCTP2 focuses on the key poverty-related infectious diseases affecting sub-Saharan Africa – HIV/AIDS, TB, malaria, lower respiratory tract infections, diarrhoeal diseases and neglected tropical diseases. As infections are rarely experienced on their own, co-infections and co-existing health conditions (including interaction with non-communicable diseases) are a further important priority.
EDCTP2 also has a focus on infectious diseases of epidemic potential, including Ebola and COVID-19. It supports international consortia that are developing the capacity of countries to prepare for and respond promptly to infectious disease outbreaks, and to undertake clinical research in outbreak situations.
The response
A partnership of equals between European and sub-Saharan Africa countries, and supported by the European Union, EDCTP2 invests in international research collaborations carrying out clinical trials of new interventions against poverty-related infectious diseases in sub-Saharan Africa.
It aims to provide the evidence to guide informed decision-making on the introduction of new interventions, and also to build the capacity of African countries to plan, undertake and lead clinical studies of local priority infections.
EDCTP2 niche
EDCTP2 occupies a distinct global niche. Concerted global efforts have seen much progress made in early-stage drug discovery and intervention development. An increasing bottleneck is the later-stage evaluation of interventions among target populations (phase II and III clinical trials) and post-licensing implementation studies (phase IV trials) to ensure smooth introduction of new interventions and to generate evidence on their performance in real-life settings – essential information for local policymakers.
EDCTP2 also has a strong emphasis on building the capacity for clinical research in sub-Saharan Africa, by supporting the development of up-and-coming researchers and scientific leaders, and by providing funds for laboratory equipment and other clinical research essentials. As well as being an integral part of clinical trial funding, support for capacity building is also provided through specific fellowship and capacity-building grants.
EDCTP2 also aims to build an enabling environment for clinical research, by strengthening national regulatory and ethical review capabilities and promoting harmonisation of approaches across the region.
As well as partnerships between EU and sub-Saharan researchers and institutions, EDCTP2 also encourages global networking, with active involvement of teams from the third countries such as USA, other high-income countries and the global South. It also supports regional networks within Africa to promote the local dissemination of knowledge and expertise. EDCTP2 also works with an extensive range of global partners to coordinate efforts, align priorities and maximise impact.


scroll down
Innovative HIV, malaria and TB vaccines
New drugs to shorten TB treatment
Broadly neutralising antibodies to
prevent HIV
Single-dose cures for malaria
Transmission-blocking vaccines for malaria
Preparation for testing of Lassa fever vaccine
•
•
•
•
•
•
index


The CHAPAS-1 trial evaluated a new fixed-dose combination containing three antiretroviral drugs – stavudine, lamivudine and nevirapine – in a formulation designed specifically for children. Its key aim was to analyse how the active ingredients in the new tablets were metabolised by children, to ensure that drug concentrations in the body would be high enough to control HIV but not so high that they would cause serious side effects.
“
crucial in
widening African
children’s access
to antiretrovirals
”


The project

The later CHAPAS-3 trial compared the efficacy and safety of three fixed-dose combinations including two without stavudine (found to have some long-term side effects in adults, leading to a recommendation that its use be discontinued in children). The trial the first of its kind in Africa studied nearly 500 children at four sites in two African countries.

The CHAPAS studies have been highly influential.CHAPAS-1 data contributed to the approval ofspecific HIV medicines (Triomune Baby/Junior)by the US Food and Drug Administration in 2007.The development of fixed-dose combinations was crucial in widening African children’s accessto antiretrovirals provided through initiativessuch as the US President’s Emergency Plan forHIV/AIDS Relief (PEPFAR) and the Clinton HIV/AIDS Initiative. The study also informed WHO recommendations on optimal drug
“
crucial in
widening African
children’s access
to antiretrovirals
”


Impact

ratios forfixed-dose combinations and on appropriatedosage according to weight.
The CHAPAS-3 trial confirmed the effectiveness of fixed-dose combinations, providing further impetus to the rollout of antiretrovirals to children. Its evidence on abacavir informed the WHO recommendation of abacavir-containing combinations for first-line therapy in children. Trial data have also been used to support applications for regulatory approval for new scored efavirenz tablets.

EDCTP2: International partnerships against
infectious disease
Dear EDCTP stakeholders,
The seventh year of implementation of the second programme of the European & Developing Countries Clinical Trials Partnership (EDCTP2) presents an opportune time to share with you the progress and achievements so far. This programme is advancing the development of diagnostics, drugs and vaccines against the most important infectious diseases affecting sub-Saharan Africa, particularly benefiting vulnerable groups such as infants, children, adolescents and pregnant women.
Dr Michael Makanga, Executive Director
We are very appreciative for the efforts of many of you who are associated with the EDCTP journey.
Kind regards,
Michael Makanga, Executive Director
The challenge
Infectious diseases remain a major cause of death and disability in sub-Saharan Africa. Despite much progress, diseases such as HIV/AIDS, malaria and tuberculosis (TB) still kill millions every year. As well as their effects on individuals, infectious diseases have a huge economic impact, in treatment costs and lost productivity.
Controlling infectious disease will therefore be core to achieving Sustainable Development Goal 3 – ensuring healthy lives and promoting wellbeing for all at all ages. Furthermore, ensuring healthy populations through effective infectious disease control will be central to achieving many other SDGs.
Control of infectious diseases requires diagnostics to detect them, drugs to treat them and vaccines to prevent them. As pathogens inevitably develop resistance to drugs, the world relies on an ongoing supply of new treatments. However, new product development is costly and risky – failure rates are high – and it is often not commercially viable for companies to
develop new products for infections mainly affecting low-income countries. Innovative partnerships are therefore required to advance new product development.
To determine their safety and efficacy, new products undergo extensive evaluation, in phase I, II and III trials of increasing size. However, the results of trials in high-income countries are only a partial guide to how well products perform in other settings. Genetic differences and past history of infections, for example, can influence how people respond to drugs and vaccines. Ideally, therefore, studies need to be conducted in sub-Saharan Africa to generate locally relevant evidence.
In addition, trials undertaken to secure regulatory approval often exclude certain populations, such as children, pregnant women or people with other infections. Further studies are needed to ensure that new products are safe, effective and can be delivered in suitable formulations to these special groups.
EDCTP2 at a glance…
Duration: 2014–2024
Funding to date: €752.74 M*
Membership and reach: 16 sub-Saharan African countries are a member of EDCTP Association; 41 African countries and 244 African institutions are participating in EDCTP projects
Studies: Funded 274 clinical studies, including 124 clinical trials
Capacity-building: We are supporting 196 African researchers through fellowships; 46 ethics and regulatory projects involving 37 African countries
*As of December 2020.
The response
A partnership of equals between EU and sub-Saharan Africa countries, EDCTP2 supports international research collaborations carrying out clinical trials of new interventions against poverty-related infectious diseases in sub-Saharan Africa.
It aims to provide the evidence to guide informed decision-making on the introduction of new interventions, and also to build the capacity of African countries to plan, undertake and lead clinical studies of local priority infections.

EDCTP2 targets
EDCTP2 focuses on the key poverty-related infectious diseases affecting sub-Saharan Africa – HIV/AIDS, TB, malaria, lower respiratory tract infections, diarrhoeal diseases and neglected tropical diseases. As infections are rarely experienced on their own, co-infections and co-existing health conditions (including interaction with non-communicable diseases) are a further important priority.
EDCTP2 also has a focus on infectious diseases of epidemic potential, including Ebola and COVID-19. It supports international consortia that are developing the capacity of countries to prepare for and respond promptly to infectious disease outbreaks, and to undertake clinical research in outbreak situations.
EDCTP2 niche
EDCTP2 occupies a distinct global niche. Concerted global efforts have seen much progress made in early-stage drug discovery and intervention development. An increasing bottleneck is the later-stage evaluation of interventions among target populations (phase II and III clinical trials) and post-licensing implementation studies (phase IV trials) to ensure smooth introduction of new interventions and to generate evidence on their performance in real-life settings – essential information for local policymakers.
EDCTP2 also has a strong emphasis on building the capacity for clinical research in sub-Saharan Africa, by supporting the development of up-and-coming researchers and scientific leaders, and by providing funds for laboratory equipment and other clinical research essentials. As well as being an integral part of clinical trial funding, support for capacity building is also provided through specific fellowship and capacity-building grants.
EDCTP2 also aims to build an enabling environment for clinical research, by strengthening national regulatory and ethical review capabilities and promoting harmonisation of approaches across the region.
As well as partnerships between EU and sub-Saharan researchers and institutions, EDCTP2 also encourages global networking, with active involvement of teams from the third countries such as USA, other high-income countries and the global South. It also supports regional networks within Africa to promote the local dissemination of knowledge and expertise. EDCTP2 also works with an extensive range of global partners to coordinate efforts, align priorities and maximise impact.
EDCTP2 highlights to date:
- New drugs for malaria, including new options for pregnant women and severely ill children.
- Novel antiretroviral drugs and innovative ways of using them, for groups such as children, adolescents and pregnant women.
- New drugs to shorten treatment of TB.
- New approaches to HIV prevention, including use of broadly neutralising antibodies.
- Extending the use of treatments against neglected infectious diseases, including parasitic worm infections, schistosome infections and leprosy.
- Practical studies of TB diagnostics and biomarkers for tracking responses to TB treatment.
- Optimising antibiotic treatment of childhood pneumonia.
- Vaccines against deadly gut infections, including enterotoxigenic E. coli and Shigella.
- Innovative vaccines for TB, HIV and malaria.
- Preparing the ground for testing of a Lassa fever vaccine, in collaboration with other partners such as the Coalition for Epidemic Preparedness and Innovations (CEPI) and Africa Centre for Disease Control (Africa CDC).
Pioneering projects funded through EDCTP2 include studies on:

Noval antiretroviral drugs for children, adolescents and pregnant woman
Antimalarial drugs for malaria prevention in pregnant women
Extending use of drugs against neglected infectious diseases, inc. parasitic worm infections
Adapting pre-exposure prophylaxis
for adolecents
Second-Line antiretroviral therapy
for children
•
•
•
•
•
Thypoid conjugate vaccine
Ebola vaccine use in children
Novel drug combinations for malaria
New TB diagnostics
Simplified treatment of cryptococcal meningitis in HIV patients
Optimising treatment of severe pneumonia in children
•
•
•
•
•
•
Innovative HIV, malaria and TB vaccines
New drugs to shorten TB treatment
Broadly neutralising antibodies to
prevent HIV
Single-dose cures for malaria
Transmission-blocking vaccines for malaria
Preparation for testing of Lassa fever vaccine
•
•
•
•
•
•
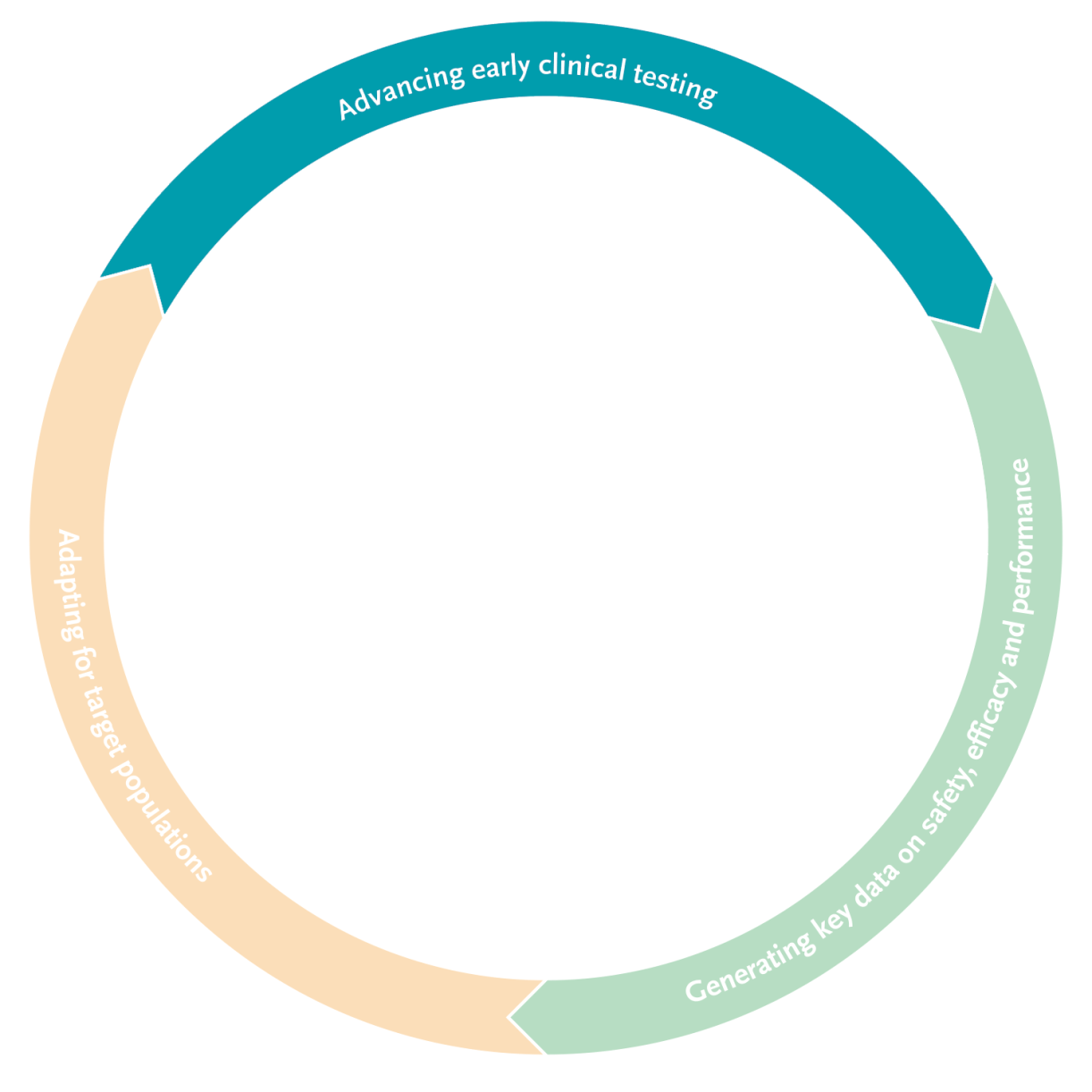
Advancing early clinical testing
Generating key data on safety, efficacy and performance
Adapting for target populations