MMV and Novartis have also been collaborating on the development of a new antimalarial treatment, ganaplacide. Although current artemisinin combination therapy (ACT) is highly effective, there are worrying signs that malaria parasites are becoming less susceptible to its effects.
Ganaplacide is an entirely new agent with a novel mechanism of action. In a phase IIb trial in Burkina Faso, Côte d’Ivoire, Gabon, Kenya, Mali and Uganda, involving the EDCTP2-funded WANECAM2 Consortium, the project team found that a combination of ganaplacide and lumefantrine was as efficacious as artemether–lumefantrine, cleared parasites just as quickly, and was well-tolerated.
These positive findings are an important step towards a new class of antimalarial to address the rising tide of resistance to existing treatments.
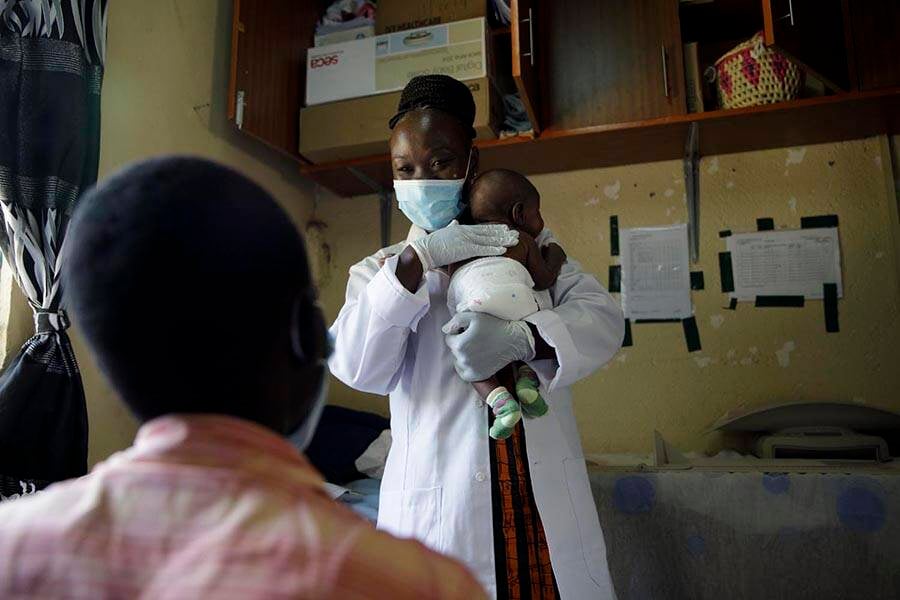
Three-quarters of malaria deaths occur in young children under the age of 5 years. However, there is no antimalarial treatment tailored to very young children, those weighing less than 5 kg. Instead, they receive a fraction of the standard dose. However, due to the immaturity of their metabolism, there are concerns that this may lead to harmfully high levels of the active compounds, artemether and lumefantrine.
With EDCTP2 funding, the PAMAfrica project, which includes the Medicines for Malaria Venture (MMV) and Novartis, has developed a new formulation of artemether–lumefantrine specifically tailored to babies and children weighing less than 5 kg. In the CALINA trial in Burkina Faso, the Democratic Republic of the Congo, Kenya, Mali, Nigeria and Zambia, the PAMAfrica team showed that the new formulation led to bloodstream levels of the drugs known to be highly effective at killing malaria parasites in older children and raised no safety concerns. This suggests that the new formulation will be efficacious and safe to use in young children.
The results were presented at the Multilateral Initiative on Malaria (MIM Society) Eighth Pan-African Malaria Conference in Kigali, Rwanda, in April 2024 and have been submitted for regulatory review.
scroll down
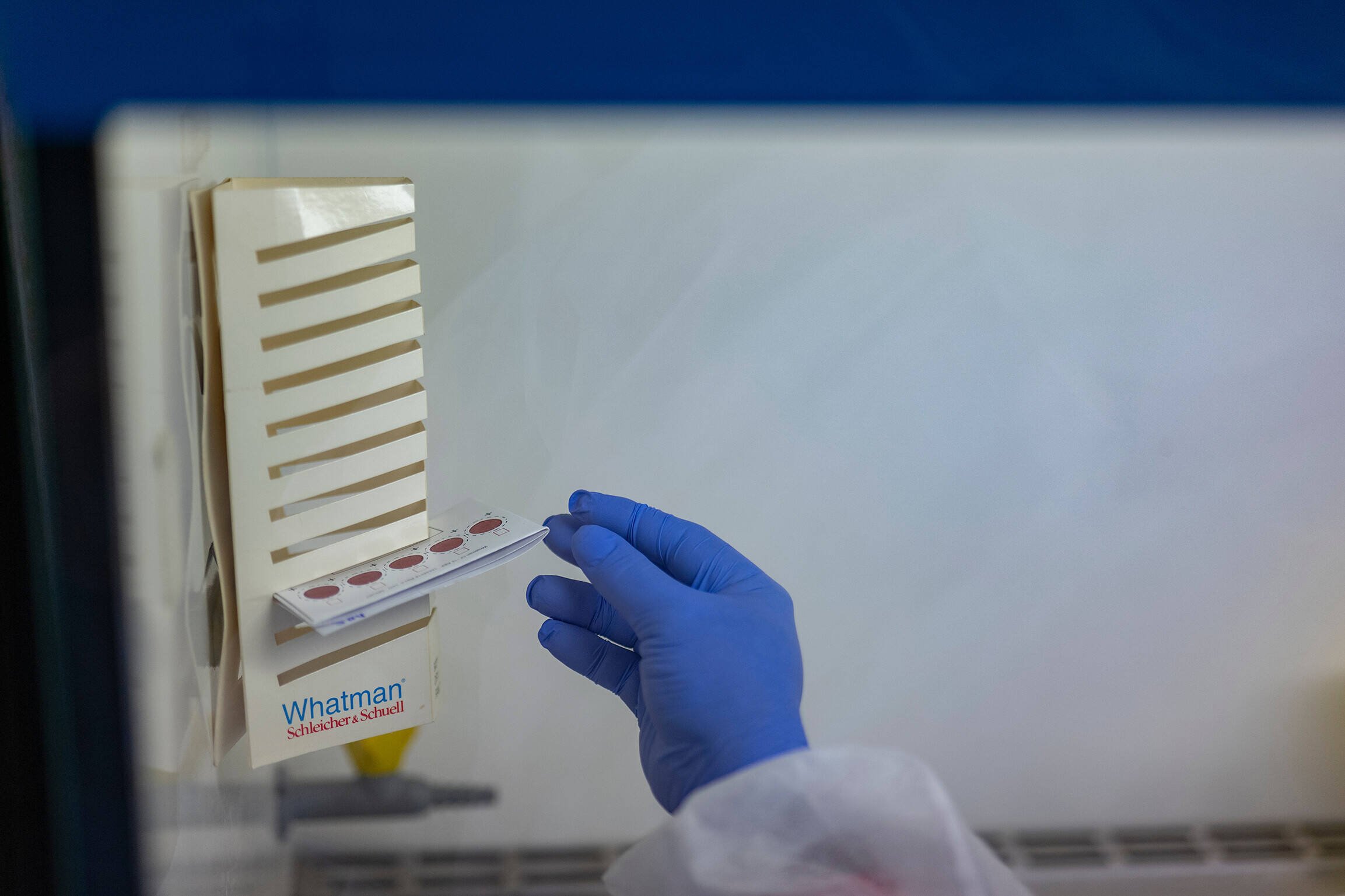
Although multiple highly effective antimalarial treatments exist, they are not always suitable for those most in need, such as pregnant women and young children.
Pregnant women are at particular risk of malaria infection, which can affect the health of both mothers and their unborn children. Prophylactic treatment with antimalarial drugs is recommended during pregnancy (intermittent preventive treatment in pregnancy, IPTp). However, the standard antimalarial drug combination used, sulfadoxine–pyrimethamine (SP), cannot be taken by women living with HIV who are on antiretroviral therapy and taking cotrimoxazole to prevent bacterial infections, as SP interferes with the action of cotrimoxazole.
To address this gap, the EDCTP2-funded MAMAH project has been evaluating the use of dihydroartemisinin–piperaquine (DPQ) as an alternative to SP. A trial involving more than 650 pregnant women living with HIV in Gabon and Mozambique found that those receiving DPQ were less likely to experience malaria infections than those who did not: six episodes of malaria were seen in the control group versus one in the DPQ group.
Use of DPQ raised no safety concerns and had no impact on birth outcomes, and no effects were seen on the risk of mother-to-child transmission of HIV. Results were equally positive for women taking different kinds of antiretroviral therapy.
The findings suggest that DPQ is suitable for use in IPTp for women with HIV infections, who currently experience a million episodes of malaria a year.
EDCTP2-funded projects have advanced antimalarial treatments for vulnerable groups, including pregnant women with HIV and young children.
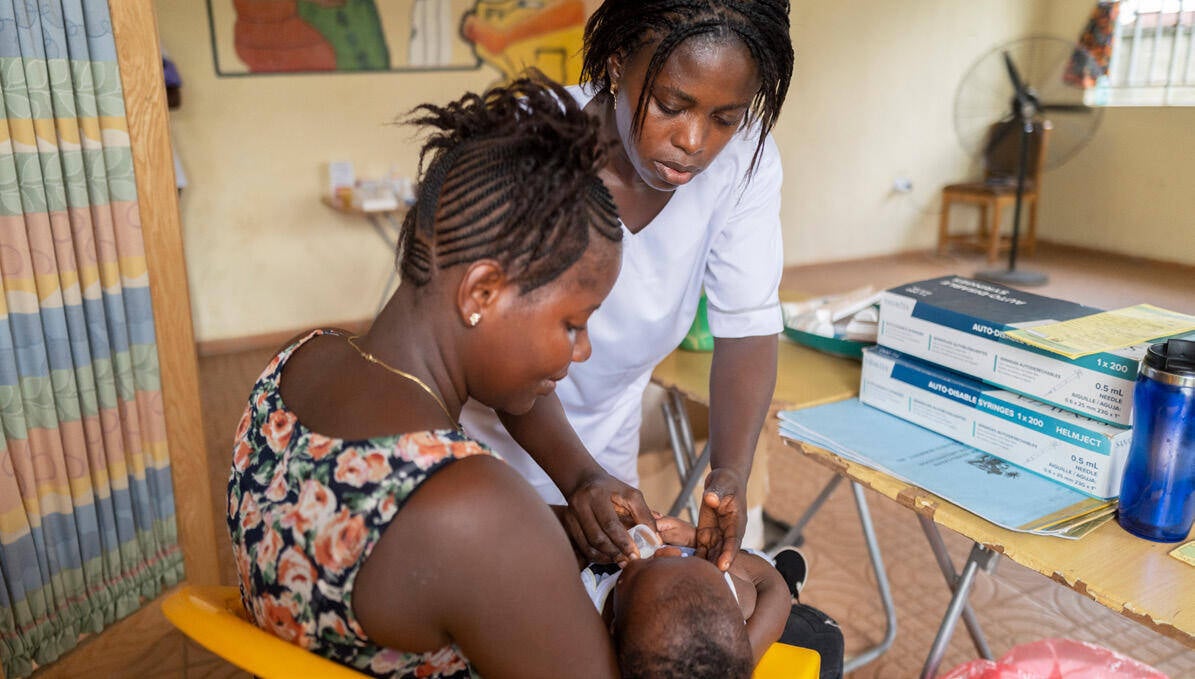
Extending antimalarial use to additional groups