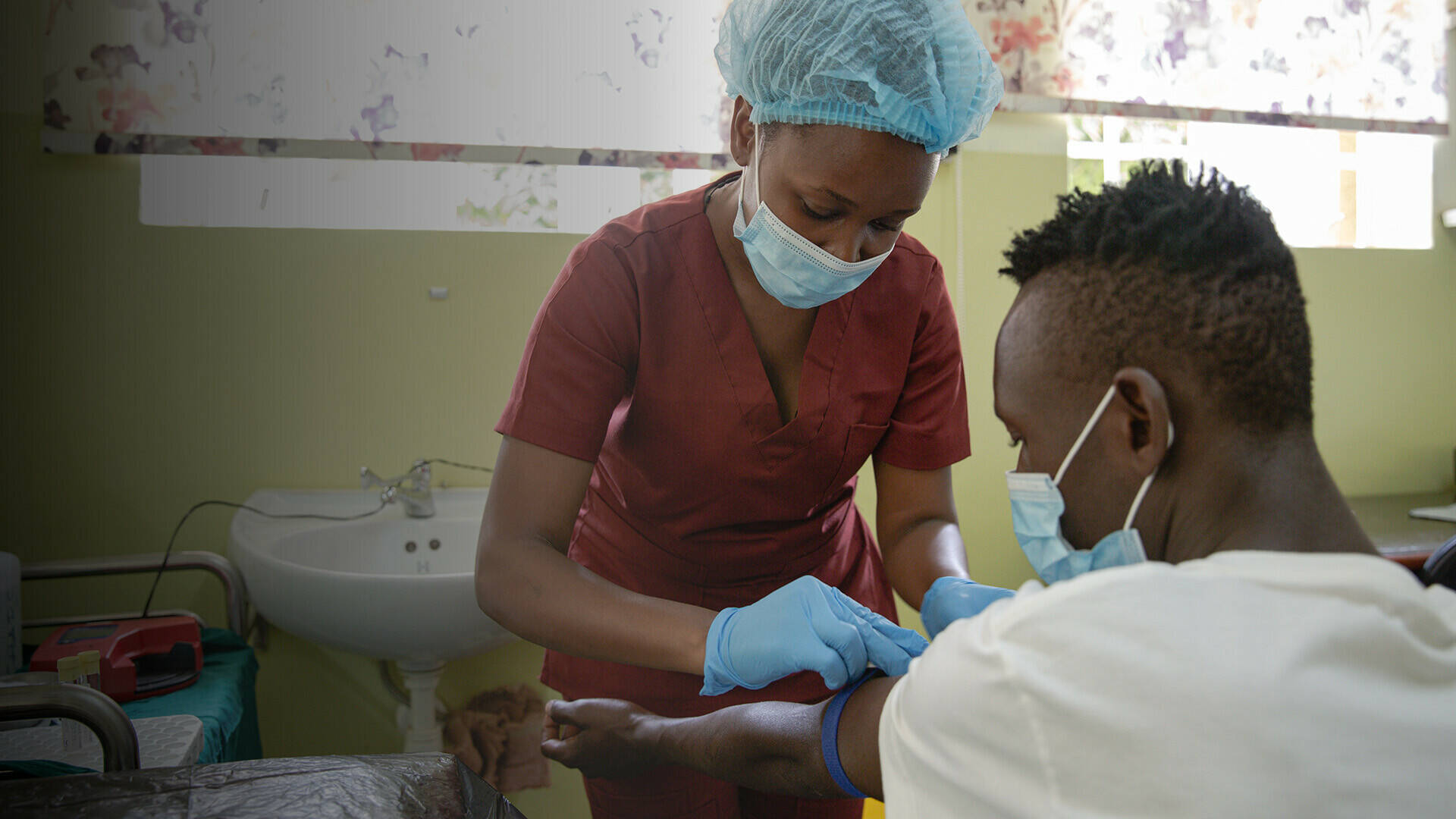
Infectious diseases remain a major cause of death and disability in sub-Saharan Africa. Controlling infectious disease will therefore be core to achieving Sustainable Development Goal 3 – ensuring healthy lives and promoting wellbeing for all at all ages. Control of infectious diseases requires diagnostics to detect them, drugs to treat them, and vaccines and novel therapies to prevent them.

Case study 04
Advancing the development and implementation of malaria vaccines
Case study 10
Boosting outbreak preparedness
Case study 08
Rolling back sleeping sickness
Case study 06
Gathering field data on TB diagnostics
Case study 05
Malaria prevention in women living with HIV
Case study 02
Improving management of meningitis in HIV-infected adults
Case study 09
Eliminating sleeping sickness in Côte d’Ivoire
Case study 07
Bringing schistosomiasis treatment to preschool-aged children
Case study 03
Treatment for children with HIV
Case study 01
Simplified treatment for a key opportunistic infection
EDCTP’s largest investments are awarded to collaborative clinical research on a range of medical interventions, especially diagnostics, drugs, and vaccines for treatment and prevention. These grants support phase I–III safety and efficacy studies, with emphasis on phase II and III trials. Phase IV pharmacovigilance and post-licensing effectiveness studies (pragmatic trials), and product-focused implementation studies are also supported.
Since 2014, EDCTP has invested €691.55 million (84% of total grant funding) to support research and development through clinical trials and product-focused implementation studies.
Of the 371 clinical studies supported by EDCTP2, the majority (228 or 61%) involve clinical trials and interventional studies of drugs, vaccines, broadly neutralising antibodies, diagnostics as well as other interventions. The remaining 39% of studies comprise non-interventional studies (e.g. observational, cohort, epidemiological, and other studies). Looking at all 155 clinical trials involving study phases, 101 (65%) of these are phase II and III trials, to determine the safety and efficacy of new or improved medical interventions, while 25 (16%) of trials are post-licensing stage (phase IV), including product-focused implementation studies. This is consistent with the strategic emphasis of the second programme on supporting phase II and III trials.
Likewise, a key feature of EDCTP2 is a focus on populations that are often excluded from clinical research but have major unmet medical needs. Of the 371 clinical studies supported, 14% involve pregnant and lactating women and their children, 26% involve newborns and infants, and 35% involve children, including adolescents. Furthermore, studies include at-risk groups such as people living with HIV, or other co-infections and co-morbidities, elderly, healthcare, as well as populations classified by WHO as key populations and vulnerable groups (e.g., commercial sex workers).
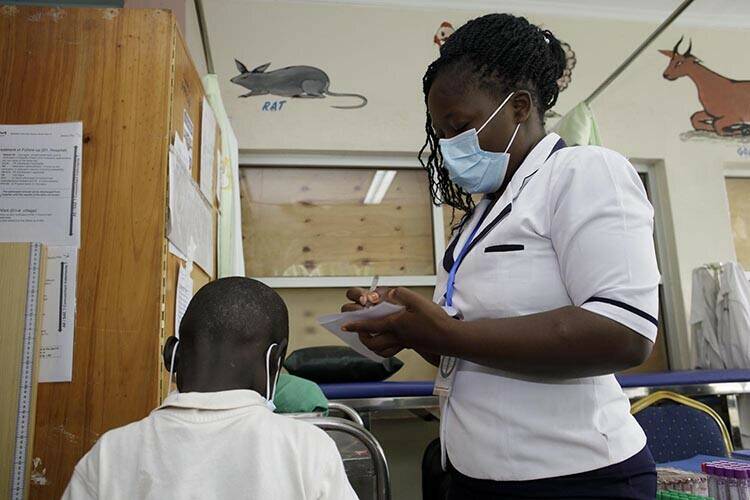
EDCTP places a strong emphasis on the importance of research use in health policy making and utilisation of research in health practice. The programme ensures that its Strategic Research Agenda reflects the priorities of sub-Saharan African countries and that research studies address questions of direct relevance to policy and practice in the region. The second interim evaluation of EDCTP2 (2023) concluded that EDCTP2 “has established open and transparent processes for consulting relevant experts and entities regarding the key priorities, which helps to ensure the continued relevance of its disease portfolio”.
Through supporting collaborative research conducted by European-African consortia, several EDCTP-supported projects have contributed to revisions of international guidelines and are impacting health practice in HIV and HIV-associated infections, malaria, TB as well as several neglected infectious diseases. At the same time, many other projects are still expected to make such contributions.
One of the key objectives of the second EDCTP programme (EDCTP2, 2014-2024) is to support high-quality clinical research that accelerates the development of medical interventions with the potential to make a real difference to the lives of millions of people across sub-Saharan Africa. Although most EDCTP2 projects are still ongoing, several projects are already generating data of direct relevance to the key poverty-related infectious diseases affecting the region, accelerating progress along the clinical development pathway, and influencing national and global policy.
scroll down
Infectious diseases remain a major cause of death and disability in sub-Saharan Africa. Controlling infectious disease will therefore be core to achieving Sustainable Development Goal 3 – ensuring healthy lives and promoting wellbeing for all at all ages. Control of infectious diseases requires diagnostics to detect them, drugs to treat them, and vaccines and novel therapies to prevent them.
scroll down

