The recent World Health Organization (WHO) recommendation of widespread use of the RTS,S/AS01 (RTS,S) malaria vaccine among children in sub-Saharan Africa and in other regions with moderate to high P. falciparum malaria transmission marked an important milestone. For over a century, the research community has been looking for a vaccine against malaria and following the positive market authorization by EMA in 2015, WHO has now recommended use of RTS,S following the results of the pilot implementation programme.

Michelle Helinski
New horizons for malaria vaccines


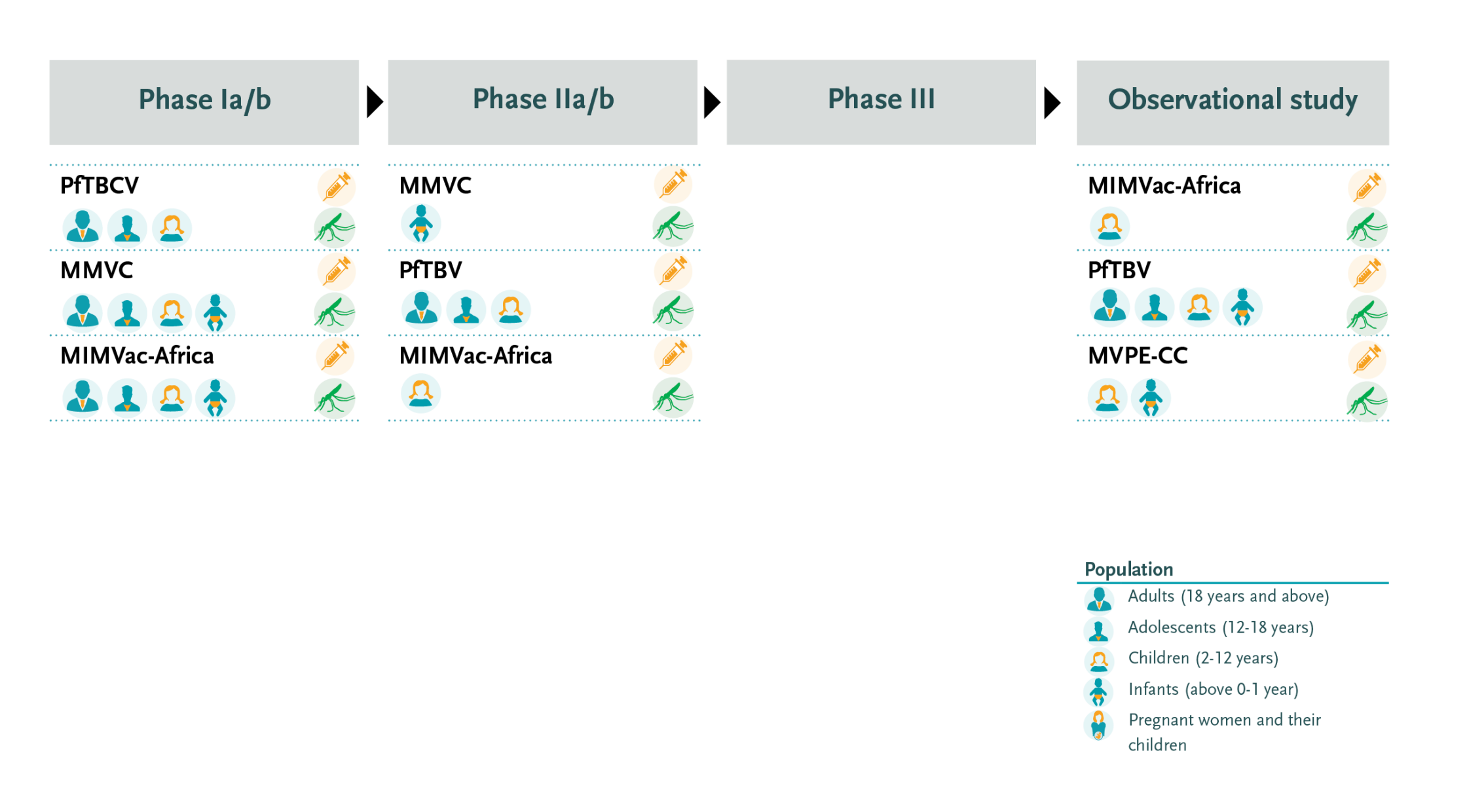
Overview of collaborative clinical research on malaria vaccine supported by EDCTP2
The MVPE-CC consortium is led by Dr Kwaku Poku Asante from the Kintampo Health Research Centre in Ghana with partners in Germany, United Kingdom, Kenya, Malawi and the United States. The project is conducting two case-control studies - embedded in the WHO-coordinated pilot implementation programme on RTS,S - to strengthen the evaluation of safety and effectiveness (including the incremental effectiveness of a 4-dose vs 3-dose regimen). In addition, the project will develop a toolkit for use by Ministries of Health and other stakeholders to support the conduct, analysis, and interpretation of case-control studies after malaria vaccine introduction and engage with these stakeholders to promote wider understanding and use of the methods.
EDCTP supports three large portfolio grants on malaria vaccine development. The projects are testing multiple candidate vaccines which target different stages of the malaria parasite. In addition, EDCTP funds an implementation study on RTS,S. Taken together, these four studies represent an investment of €48.1 million.
In addition to the MMVC project, EDCTP supports the following collaborative clinical trials and clinical studies on malaria vaccine:
The MIMVaC-Africa consortium conducts clinical trials on a portfolio of five malaria vaccine candidates: three pre-erythrocytic vaccine candidates and two blood-stage candidates. The consortium is led by Dr Sodiomon Sirima of the Groupe de Recherche Action en Santé (GRAS) in Burkina Faso, with partners in Gabon, Germany, Japan, Luxembourg, Mozambique, the Netherlands, Tanzania, and the United Kingdom.
It will leverage its recently developed capacity to work with the controlled human malaria infection (CHMI) challenge model. With the CHMI model enabling rapid testing and comparison of the vaccine candidates in Europe and Africa, the most promising candidates will be selected for field efficacy trials in Africa. The MIMVaC-Africa programme will also strengthen the research capacity at clinical study sites.
The PfTBV consortium is coordinated by Dr Issaka Sagara of the University of Sciences, Techniques and Technologies in Bamako, Mali, with partners in Burkina Faso, Denmark, Guinea, Liberia, Mali, the Netherlands and the United States. The consortium is evaluating a portfolio of three innovative candidate malaria vaccines that aim to block transmission of the parasite. Recent results from a phase II community trial on the transmission-blocking vaccine candidate Pfs230D1-EPA-AS01 showed excellent efficacy and safety in Malian children and adults.
“A few days ago, the World Health Organization approved for the first time the use of a malaria vaccine for children in sub-Saharan African countries and other countries heavily affected by this disease. This vaccine was developed thanks to the strong direct and indirect involvement of the EDCTP,” said Member of the European Parliament and lead negotiator of the Committee on Industry, Research, and Energy (ITRE) for the Joint Undertakings under Horizon Europe, Maria da Graça Carvalho.
In addition, EDCTP is currently supporting the MVPE-CC project that is collecting additional data from the RTS,S pilot implementation programme through embedding two case control studies that will gather additional information on severe malaria and safety. The case-control studies take place in Malawi, Kenya and Ghana.
Over the years, the EDCTP programme has made important contributions to the development of the RTS,S vaccine through our support to institutional and individual capacity strengthening activities aimed to strengthen the research environment for clinical trials in sub-Saharan Africa. This support was acknowledged in a recent European Parliament session where members voted in favour of the establishment of the Global Health EDCTP 3 Joint Undertaking by adopting a legislative proposal on Joint Undertakings under Horizon Europe. During the plenary debate, the work of EDCTP was cited as an example of the importance of the proposal:
The MMVC project is led by Professor Adrian Hill from Oxford University, United Kingdom with partners in Burkina Faso, France, Germany, India, the Netherlands, Sierra Leone, Sweden, Tanzania, The Gambia and the United Kingdom.
The project aims to develop the first ever combination multi-stage malaria vaccine designed to target different stages of the Plasmodium falciparum parasite’s lifecycle. This ambitious project encompasses a series of tightly coordinated lead-in trials. The front runner component is the R21/Matrix-M candidate vaccine.
One of the leading candidates is the R21/Matrix-M candidate from the University of Oxford/Serum Institute of India. This candidate recently obtained highly promising results in an EDCTP-supported Phase 2b trial conducted in Burkina Faso as part of the Multi-Stage Malaria Vaccine Consortium (MMVC). In this trial, children aged 5-17 months were randomised to receive either the R21/Matrix-M (MM) candidate (low- or high-dose) or a control vaccine (rabies). A vaccine efficacy of 77% against clinical malaria was seen in the higher-dose adjuvant group after 12 months of follow-up, with 71% reported in the lower-dose adjuvant group, with no serious adverse events attributed to the vaccine observed (read full publication).
scroll down
The WHO special session at the EDCTP Forum concluded with Dr Pedro Alonso, Director of the WHO Global Malaria Programme, urging the malaria community to work together - using all available medical interventions in the malaria prevention and control toolbox - to control the disease and further reduce malaria morbidity and mortality. The EDCTP2 programme is making significant investments towards malaria research & development through project and portfolio funding, and its successor the Global Health EDCTP3 programme is committed to continue our support towards this goal.
WHO reflected on this important milestone in a special session at the recent EDCTP Forum in Maputo, Mozambique, where results from the pilot implementation programme in Ghana, Kenya and Malawi were presented. The session also covered other promising malaria vaccine candidates and novel approaches, including from the current portfolio of EDCTP-supported projects.
scroll down
The recent World Health Organization (WHO) recommendation of widespread use of the RTS,S/AS01 (RTS,S) malaria vaccine among children in sub-Saharan Africa and in other regions with moderate to high P. falciparum malaria transmission marked an important milestone. For over a century, the research community has been looking for a vaccine against malaria and following the positive market authorization by EMA in 2015, WHO has now recommended use of RTS,S following the results of the pilot implementation programme.
New horizons for malaria vaccines
Michelle Helinski

Next steps to further community engagement
To consolidate its work in community engagement, EDCTP has created a working group involving experts from the General Assembly and the Scientific Advisory Committee. Currently, the working group is reviewing EDCTP-funded research through the lens of community engagement. It will develop recommendations to strengthen EDCTP’s strategy in this area, taking into consideration the orientation of the future third EDCTP programme, i.e. its expected increased focus on late-stage product development, vulnerable populations and emerging and re-emerging infections.
WHO reflected on this important milestone in a special session at the recent EDCTP Forum in Maputo, Mozambique, where results from the pilot implementation programme in Ghana, Kenya and Malawi were presented. The session also covered other promising malaria vaccine candidates and novel approaches, including from the current portfolio of EDCTP-supported projects.
The WHO special session at the EDCTP Forum concluded with Dr Pedro Alonso, Director of the WHO Global Malaria Programme, urging the malaria community to work together - using all available medical interventions in the malaria prevention and control toolbox - to control the disease and further reduce malaria morbidity and mortality. The EDCTP2 programme is making significant investments towards malaria research & development through project and portfolio funding, and its successor the Global Health EDCTP3 programme is committed to continue our support towards this goal.

Community engagement in
clinical research
Through its various funding mechanisms, EDCTP has invested in a range of community engagement activities. The international collaborative clinical research projects usually comprise an element of community engagement such as through stakeholder meetings with community representatives to ensure their active involvement throughout the study. Feedback events and other engagement activities are also planned. Many of the clinical research sites where EDCTP-funded trials take place have longstanding engagement with local stakeholders through Community Advisory Boards.
An example of such a collaborative approach between researchers and the community is the EMPIRICAL study: ‘Empirical treatment against cytomegalovirus and tuberculosis in severe pneumonia in HIV-infected infants: a randomised controlled clinical trial’. Particular attention is paid to ensuring that participants understand the purpose and procedures of the clinical trial, as well as involving peer counsellors in post-test counselling to provide support to caregivers regarding their HIV diagnosis and to explain the voluntary nature of research studies.
Collaboration with a Community Advisory Board at each clinical research site is integrated in the study approach, including establishing a local board if one is not already available. The Community Advisory Boards will contribute to increase awareness and develop trust between the research teams and the HIV community. They will aim to dispel any myths and rumours that may have arisen due to a lack of information or an understanding according to local beliefs. To do so, the boards will include key representatives from the community, such as volunteers from activist and patient groups and representatives from (non-)governmental organisations that best represent the infants and families affected by the transmission of HIV-infection. Moreover, local authorities such as traditional chiefs and city representatives will be informed about the trial.
The Community Advisory Boards will be convened before submission of the study protocol to the local Ethics Committee. They will provide guidance and their perspective on the protocol, including what must be addressed during post-test counselling. Moreover, they will provide feedback on the informed consent forms and the study implementation, and assist in the dissemination of research results. Biannual meetings will be held with the boards to ensure a dialogue throughout the trial, to identify, discuss and resolve issues arising. At the end of the study, participants and families will be informed that the trial will be closing. When the trial results are available, an information meeting will take place involving every trial participant and their care givers, the Community Advisory Board and local authorities.
One of the leading candidates is the R21/Matrix-M candidate from the University of Oxford/Serum Institute of India. This candidate recently obtained highly promising results in an EDCTP-supported Phase 2b trial conducted in Burkina Faso as part of the Multi-Stage Malaria Vaccine Consortium (MMVC). In this trial, children aged 5-17 months were randomised to receive either the R21/Matrix-M (MM) candidate (low- or high-dose) or a control vaccine (rabies). A vaccine efficacy of 77% against clinical malaria was seen in the higher-dose adjuvant group after 12 months of follow-up, with 71% reported in the lower-dose adjuvant group, with no serious adverse events attributed to the vaccine observed (read full publication).

Overview of collaborative clinical research on malaria vaccine supported by EDCTP2
The MVPE-CC consortium is led by Dr Kwaku Poku Asante from the Kintampo Health Research Centre in Ghana with partners in Germany, United Kingdom, Kenya, Malawi and the United States. The project is conducting two case-control studies - embedded in the WHO-coordinated pilot implementation programme on RTS,S - to strengthen the evaluation of safety and effectiveness (including the incremental effectiveness of a 4-dose vs 3-dose regimen). In addition, the project will develop a toolkit for use by Ministries of Health and other stakeholders to support the conduct, analysis, and interpretation of case-control studies after malaria vaccine introduction and engage with these stakeholders to promote wider understanding and use of the methods.
In addition to the MMVC project, EDCTP supports the following collaborative clinical trials and clinical studies on malaria vaccine:
The MIMVaC-Africa consortium conducts clinical trials on a portfolio of five malaria vaccine candidates: three pre-erythrocytic vaccine candidates and two blood-stage candidates. The consortium is led by Dr Sodiomon Sirima of the Groupe de Recherche Action en Santé (GRAS) in Burkina Faso, with partners in Gabon, Germany, Japan, Luxembourg, Mozambique, the Netherlands, Tanzania, and the United Kingdom.
It will leverage its recently developed capacity to work with the controlled human malaria infection (CHMI) challenge model. With the CHMI model enabling rapid testing and comparison of the vaccine candidates in Europe and Africa, the most promising candidates will be selected for field efficacy trials in Africa. The MIMVaC-Africa programme will also strengthen the research capacity at clinical study sites.
The PfTBV consortium is coordinated by Dr Issaka Sagara of the University of Sciences, Techniques and Technologies in Bamako, Mali, with partners in Burkina Faso, Denmark, Guinea, Liberia, Mali, the Netherlands and the United States. The consortium is evaluating a portfolio of three innovative candidate malaria vaccines that aim to block transmission of the parasite. Recent results from a phase II community trial on the transmission-blocking vaccine candidate Pfs230D1-EPA-AS01 showed excellent efficacy and safety in Malian children and adults.
Community engagement
for policy
The WHO special session at the EDCTP Forum concluded with Dr Pedro Alonso, Director of the WHO Global Malaria Programme, urging the malaria community to work together - using all available medical interventions in the malaria prevention and control toolbox - to control the disease and further reduce malaria morbidity and mortality. The EDCTP2 programme is making significant investments towards malaria research & development through project and portfolio funding, and its successor the Global Health EDCTP3 programme is committed to continue our support towards this goal.
Community engagement and ethics review
The EDCTP-funded projects to develop capacities for ethics review and regulatory capacities usually conduct an information meeting with stakeholders including the community. The aim of these meetings is to inform community members on research ethics and the regulatory approval process.
The HATUA-KENYA project ‘Enabling compliance and building capacity and community for clinical research in Kenya’ illustrates such engagement. In collaboration with the Bioethics Society of Kenya, it organised several workshops and events to support sharing of experiences across institutions and discuss ethics issues. The project has held open online ‘bioethics cafes’. A conference on ‘Contemporary Issues in Bioethics’, in March 2020 in Nairobi, Kenya, covered topics such as community engagement, research involving minors, and ethical issues during pandemics.