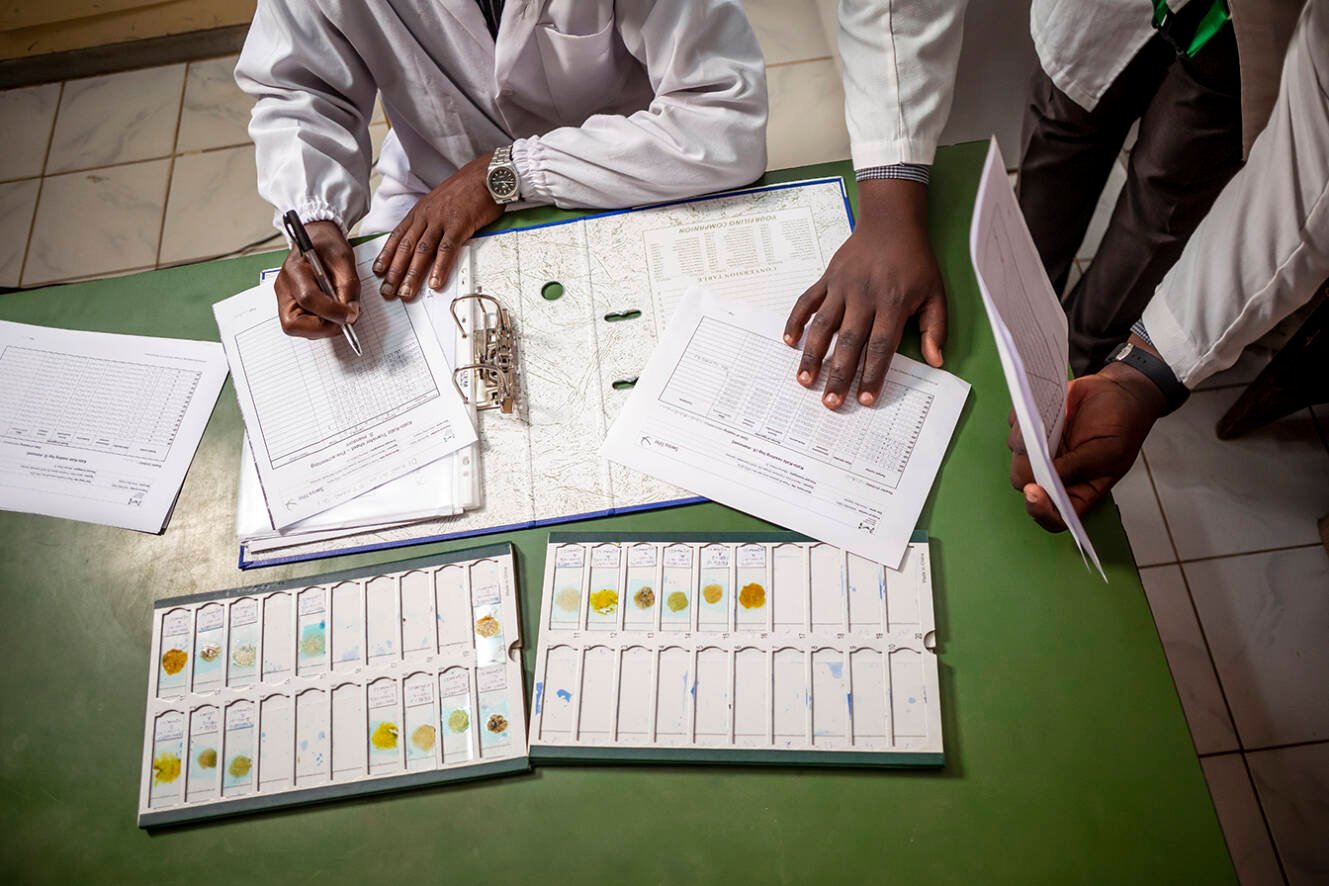
An estimated quarter of the world’s population is infected with soil-transmitted helminths, a group of parasitic worms transmitted via contaminated soil. The standard treatment for these infections is albendazole, but this drug is not effective against all species of helminth and there is growing evidence of parasite resistance (as shown for example by the EDCTP-funded PROFORMA consortium in Ethiopia).
Greater breadth of protection could come from combining albendazole with a second drug, ivermectin. In the phase II/III ALIVE trial in Ethiopia, Kenya and Mozambique, the EDCTP-funded STOP Consortium demonstrated the efficacy of a fixed-dose combination of ivermectin and albendazole for the treatment of soil-transmitted helminths in nearly 4000 children. The findings formed part of a submission to the EMA made in 2023.
The Consortium’s work has also been supported by a UK-funded Participating States Initiated Activity (PSIA). This funding enabled the project team to respond to scientific advice received from the EMA relating to the new fixed-dose combination. To avoid delays in appraisal, the EMA recommended carrying out a preparatory study to demonstrate that the two drugs given together have broadly the same activity as when given separately (a bioequivalence study) as well as a phase II safety trial in Kenya as a prelude to the main ALIVE trial.
Further development of the new fixed-dose combination will take place through the recently launched STOP2030 project, funded through the Global Health EDCTP3 programme. This project is testing the safety and effectiveness of the fixed-dose combination in mass drug administration campaigns in Kenya and Ghana.
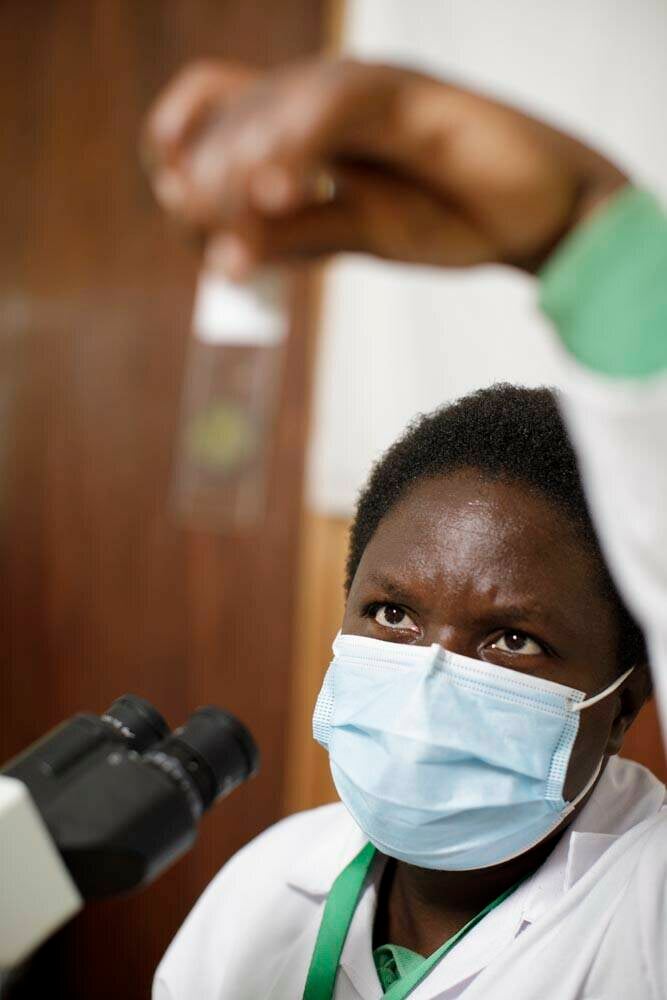
In 2023, the EMA also delivered a positive scientific opinion on fexinidazole as a treatment for sleeping sickness caused by the Trypanosoma brucei rhodesiense parasite4. Fexinidazole is already approved for the most common form of sleeping sickness (human African trypanosomiasis, HAT), caused by T. b. gambiense (gHAT). The HAT-r-ACC project showed that the drug was also effective against the less common but potentially lethal rHAT form of the disease, caused by T. b. rhodesiense, which is found in parts of East and Southern Africa. Fexinidazole is already being used in multiple countries in sub-Saharan Africa to treat gHAT, and the positive scientific opinion is a key step to extending its use to rHAT.
Through the PZQ4PSAC project, EDCTP and the Japan-based Global Health Innovative Technology (GHIT) Fund have been supporting the Paediatric Praziquantel Consortium’s development of a child-friendly version of praziquantel. This drug is used to control schistosomiasis, a parasitic worm infection that affects around 250 million people worldwide. Praziquantel is used in mass drug administration programmes to control the spread of the parasite, but is not suitable for use in children 6 years of age and younger.
EDCTP and GHIT funded a phase III trial of a new form of praziquantel, known as arpraziquantel, which dissolves in water or in the mouth, and can be given to young children. The trial showed that arpraziquantel was efficacious, safe and well-tolerated by young children. In late 2023, arpraziquantel received a positive scientific opinion from the European Medicines Agency (EMA), leading to WHO prequalification and listing of the drug on the WHO Essential Medicines List3. In parallel, EDCTP and GHIT are supporting the ADOPT project, an implementation research project that will identify optimal ways to provide access to and introduce arpraziquantel into endemic countries.Human challenge studies have also shown that the most abundant protein on the surface of merozoites, MSP-1, may be a critical target of several protective immune responses. Previous studies have tended to focus on fragments of MSP-1, but Professor Osier’s group found that antibodies to full-length MSP-1 protein correlated with protection against malaria. These antibodies trigger five different protective immune responses, each of which seems to independently contribute to protection. The results could have important implications for vaccine development targeting MSP-1, which has to date had limited success.
scroll down
Neglected infectious diseases cause untold misery while, as their name implies, generating comparatively little interest. Three success stories from 2023 show how children and adults can benefit from a focus on developing new treatments.
Three EDCTP-funded projects have made major advances in the development of treatments for neglected infectious diseases.
Not so neglected