Although the trial showed that H56:IC31 was safe, well-tolerated and capable of stimulating an immune response, it did not prevent recurrence of TB. In light of the results, development of the vaccine has been halted.
Although both sets of results are disappointing, the two trials have nonetheless made important contributions to the knowledge base and helped to build the capacity for large, high-quality trials in sub-Saharan Africa. The PrEPVacc trial will still be providing valuable data on different versions of PrEP, informing implementation efforts. In addition, the POR TB results will shed light on mechanisms of recurrence and immune responses to M. tuberculosis, and inform future TB vaccine development.
scroll down
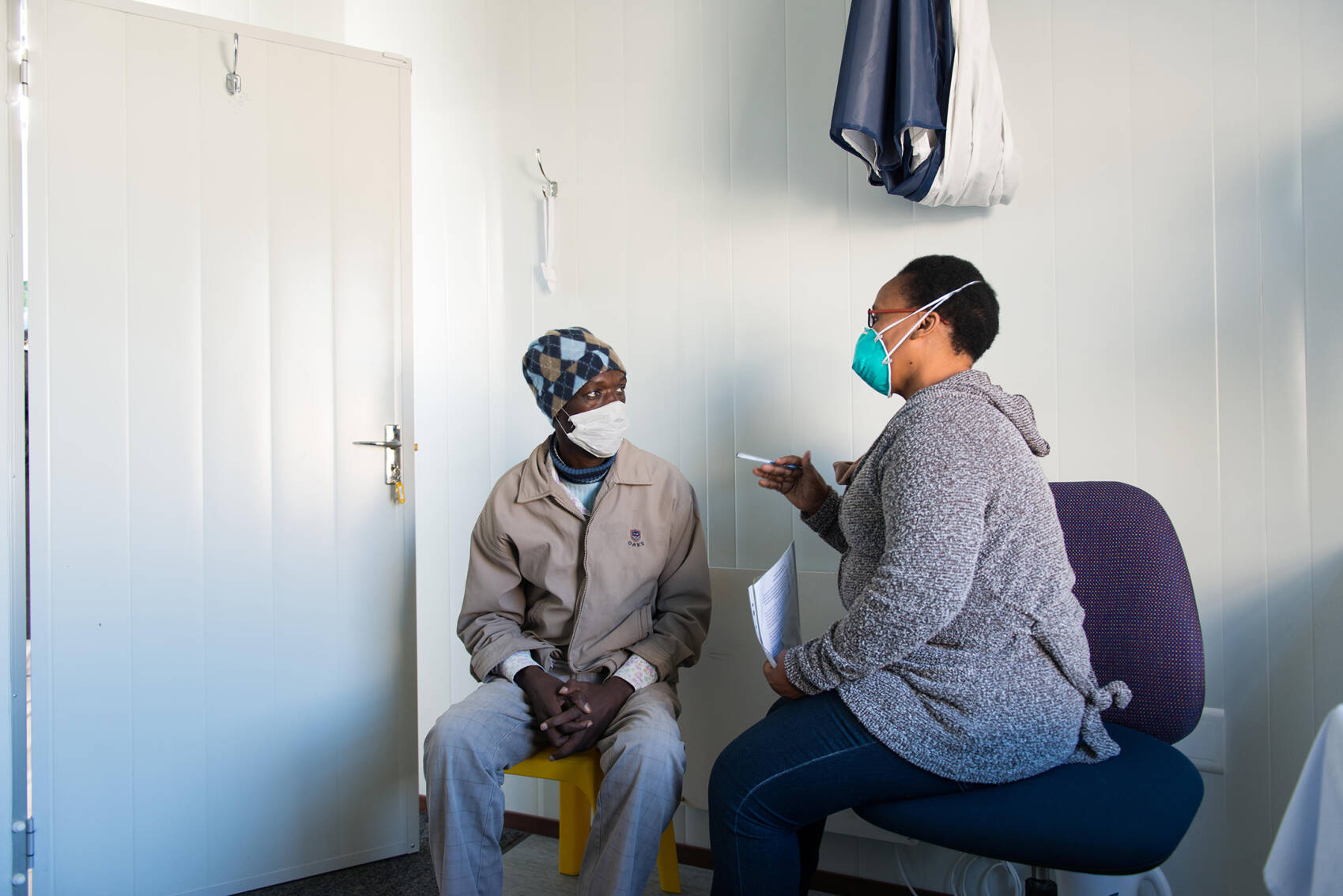
HIV and TB are the leading causes of disease in sub-Saharan Africa. Although effective treatments exist, long-term control of these pathogens is likely to require interventions able to prevent infection, such as vaccines. However, both HIV and the bacterium responsible for TB, Mycobacterium tuberculosis, present major challenges to vaccine development.
The PrEPVacc trial, funded by EDCTP and other partners, has been assessing whether two different combinations of candidate HIV vaccines, when combined with pre-exposure prophylaxis (PrEP, pre-emptive use of antiretroviral drugs to prevent infection), reduce the risk of HIV infection. In 2023, the trial’s independent data monitoring committee recommended halting use of the vaccines as there was little prospect that they would be shown to be efficacious by the end of the trial. Participants are continuing to receive the PrEP components of the intervention.
The POR TB consortium has been evaluating use of a candidate TB vaccine, H56:IC31, in a ‘prevention of recurrence’ (POR) trial in South Africa and Tanzania. After successful treatment with TB drugs, around one in ten TB patients experience recurrence of TB, either due to reinfection or re-emergence of their initial infection. The POR strategy aims to reduce this risk of recurrence.
Trials of an HIV and a TB vaccine have generated disappointing results, emphasising the huge challenges facing vaccine developers in these fields.
Vaccine disappointments – but key lessons learned